Association of Vasopressin Plus Catecholamine Vasopressors vs Catecholamines Alone With Atrial Fibrillation in Patients With Distributive Shock: A Systematic Review and Meta-analysis
- PMID: 29801010
- PMCID: PMC6583502
- DOI: 10.1001/jama.2018.4528
Association of Vasopressin Plus Catecholamine Vasopressors vs Catecholamines Alone With Atrial Fibrillation in Patients With Distributive Shock: A Systematic Review and Meta-analysis
Abstract
Importance: Vasopressin is an alternative to catecholamine vasopressors for patients with distributive shock-a condition due to excessive vasodilation, most frequently from severe infection. Blood pressure support with a noncatecholamine vasopressor may reduce stimulation of adrenergic receptors and decrease myocardial oxygen demand. Atrial fibrillation is common with catecholamines and is associated with adverse events, including mortality and increased length of stay (LOS).
Objectives: To determine whether treatment with vasopressin + catecholamine vasopressors compared with catecholamine vasopressors alone was associated with reductions in the risk of adverse events.
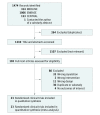
Data sources: MEDLINE, EMBASE, and CENTRAL were searched from inception to February 2018. Experts were asked and meta-registries searched to identify ongoing trials.
Study selection: Pairs of reviewers identified randomized clinical trials comparing vasopressin in combination with catecholamine vasopressors to catecholamines alone for patients with distributive shock.
Data extraction and synthesis: Two reviewers abstracted data independently. A random-effects model was used to combine data.
Main outcomes and measures: The primary outcome was atrial fibrillation. Other outcomes included mortality, requirement for renal replacement therapy (RRT), myocardial injury, ventricular arrhythmia, stroke, and LOS in the intensive care unit and hospital. Measures of association are reported as risk ratios (RRs) for clinical outcomes and mean differences for LOS.
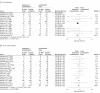
Results: Twenty-three randomized clinical trials were identified (3088 patients; mean age, 61.1 years [14.2]; women, 45.3%). High-quality evidence supported a lower risk of atrial fibrillation associated with vasopressin treatment (RR, 0.77 [95% CI, 0.67 to 0.88]; risk difference [RD], -0.06 [95% CI, -0.13 to 0.01]). For mortality, the overall RR estimate was 0.89 (95% CI, 0.82 to 0.97; RD, -0.04 [95% CI, -0.07 to 0.00]); however, when limited to trials at low risk of bias, the RR estimate was 0.96 (95% CI, 0.84 to 1.11). The overall RR estimate for RRT was 0.74 (95% CI, 0.51 to 1.08; RD, -0.07 [95% CI, -0.12 to -0.01]). However, in an analysis limited to trials at low risk of bias, RR was 0.70 (95% CI, 0.53 to 0.92, P for interaction = .77). There were no significant differences in the pooled risks for other outcomes.
Conclusions and relevance: In this systematic review and meta-analysis, the addition of vasopressin to catecholamine vasopressors compared with catecholamines alone was associated with a lower risk of atrial fibrillation. Findings for secondary outcomes varied.
Conflict of interest statement
Figures

References
-
- Landry DW, Oliver JA. The pathogenesis of vasodilatory shock. N Engl J Med. 2001;345(8):588-595. - PubMed
-
- Machado FR, Cavalcanti AB, Bozza FA, et al. ; SPREAD Investigators; Latin American Sepsis Institute Network . The epidemiology of sepsis in Brazilian intensive care units (the Sepsis Prevalence Assessment Database, SPREAD): an observational study. Lancet Infect Dis. 2017;17(11):1180-1189. - PubMed
-
- SepNet Critical Care Trials Group Incidence of severe sepsis and septic shock in German intensive care units: the prospective, multicentre INSEP study. Intensive Care Med. 2016;42(12):1980-1989. - PubMed
-
- Rhodes A, Evans LE, Alhazzani W, et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock: 2016. Crit Care Med. 2017;45(3):486-552. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

