Autoimmune Th17 Cells Induced Synovial Stromal and Innate Lymphoid Cell Secretion of the Cytokine GM-CSF to Initiate and Augment Autoimmune Arthritis
- PMID: 29802020
- PMCID: PMC6024031
- DOI: 10.1016/j.immuni.2018.04.009
Autoimmune Th17 Cells Induced Synovial Stromal and Innate Lymphoid Cell Secretion of the Cytokine GM-CSF to Initiate and Augment Autoimmune Arthritis
Abstract
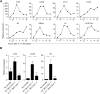
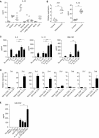
Despite the importance of Th17 cells in autoimmune diseases, it remains unclear how they control other inflammatory cells in autoimmune tissue damage. Using a model of spontaneous autoimmune arthritis, we showed that arthritogenic Th17 cells stimulated fibroblast-like synoviocytes via interleukin-17 (IL-17) to secrete the cytokine GM-CSF and also expanded synovial-resident innate lymphoid cells (ILCs) in inflamed joints. Activated synovial ILCs, which expressed CD25, IL-33Ra, and TLR9, produced abundant GM-CSF upon stimulation by IL-2, IL-33, or CpG DNA. Loss of GM-CSF production by either ILCs or radio-resistant stromal cells prevented Th17 cell-mediated arthritis. GM-CSF production by Th17 cells augmented chronic inflammation but was dispensable for the initiation of arthritis. We showed that GM-CSF-producing ILCs were present in inflamed joints of rheumatoid arthritis patients. Thus, a cellular cascade of autoimmune Th17 cells, ILCs, and stromal cells, via IL-17 and GM-CSF, mediates chronic joint inflammation and can be a target for therapeutic intervention.
Keywords: GM-CSF; IL-17; ILCs; SKG; Th17; arthritis; autoimmunity; innate lymphoid cells.
Copyright © 2018 Elsevier Inc. All rights reserved.
Figures






Comment in
-
A cellular cascade of GM-CSF production.Nat Rev Rheumatol. 2018 Jul;14(7):386. doi: 10.1038/s41584-018-0038-0. Nat Rev Rheumatol. 2018. PMID: 29891913 No abstract available.
Similar articles
-
Synovial Tissue Inflammation Mediated by Autoimmune T Cells.Front Immunol. 2019 Aug 21;10:1989. doi: 10.3389/fimmu.2019.01989. eCollection 2019. Front Immunol. 2019. PMID: 31497022 Free PMC article. Review.
-
Complementary action of granulocyte macrophage colony-stimulating factor and interleukin-17A induces interleukin-23, receptor activator of nuclear factor-κB ligand, and matrix metalloproteinases and drives bone and cartilage pathology in experimental arthritis: rationale for combination therapy in rheumatoid arthritis.Arthritis Res Ther. 2015 Jun 17;17(1):163. doi: 10.1186/s13075-015-0683-5. Arthritis Res Ther. 2015. PMID: 26081345 Free PMC article.
-
Cytokines in chronic inflammatory arthritis. VI. Analysis of the synovial cells involved in granulocyte-macrophage colony-stimulating factor production and gene expression in rheumatoid arthritis and its regulation by IL-1 and tumor necrosis factor-alpha.J Immunol. 1991 May 15;146(10):3365-71. J Immunol. 1991. PMID: 2026869
-
T cell expression of granulocyte-macrophage colony-stimulating factor in juvenile arthritis is contingent upon Th17 plasticity.Arthritis Rheumatol. 2014 Jul;66(7):1955-60. doi: 10.1002/art.38647. Arthritis Rheumatol. 2014. PMID: 24692225 Free PMC article.
-
Roles of GM-CSF in the Pathogenesis of Autoimmune Diseases: An Update.Front Immunol. 2019 Jun 4;10:1265. doi: 10.3389/fimmu.2019.01265. eCollection 2019. Front Immunol. 2019. PMID: 31275302 Free PMC article. Review.
Cited by
-
GM-CSF Primes Proinflammatory Monocyte Responses in Ankylosing Spondylitis.Front Immunol. 2020 Jul 16;11:1520. doi: 10.3389/fimmu.2020.01520. eCollection 2020. Front Immunol. 2020. PMID: 32765525 Free PMC article.
-
Acetylated Diacylglycerol 1-palmitoyl-2-linoleoyl-3-acetyl-rac-glycerol in Autoimmune Arthritis and Interstitial Lung Disease in SKG Mice.Biomedicines. 2021 Aug 27;9(9):1095. doi: 10.3390/biomedicines9091095. Biomedicines. 2021. PMID: 34572282 Free PMC article.
-
Immunoporosis: Role of Innate Immune Cells in Osteoporosis.Front Immunol. 2021 Aug 5;12:687037. doi: 10.3389/fimmu.2021.687037. eCollection 2021. Front Immunol. 2021. PMID: 34421899 Free PMC article. Review.
-
Synovial Fluid as a Crucial Component of the Joint Microenvironment in Rheumatoid Arthritis.Immune Netw. 2025 Feb 5;25(2):e2. doi: 10.4110/in.2025.25.e2. eCollection 2025 Apr. Immune Netw. 2025. PMID: 40342839 Free PMC article. Review.
-
The effect of serum IL-2 levels on the prognosis of primary biliary cholangitis-related liver failure and the preliminary exploration of its mechanism.Front Immunol. 2022 Sep 8;13:995223. doi: 10.3389/fimmu.2022.995223. eCollection 2022. Front Immunol. 2022. PMID: 36159788 Free PMC article.
References
-
- Alvaro-Gracia J.M., Zvaifler N.J., Brown C.B., Kaushansky K., Firestein G.S. Cytokines in chronic inflammatory arthritis. VI. Analysis of the synovial cells involved in granulocyte-macrophage colony-stimulating factor production and gene expression in rheumatoid arthritis and its regulation by IL-1 and tumor necrosis factor-alpha. J. Immunol. 1991;146:3365–3371. - PubMed
-
- Behrens F., Tak P.P., Østergaard M., Stoilov R., Wiland P., Huizinga T.W., Berenfus V.Y., Vladeva S., Rech J., Rubbert-Roth A. MOR103, a human monoclonal antibody to granulocyte-macrophage colony-stimulating factor, in the treatment of patients with moderate rheumatoid arthritis: results of a phase Ib/IIa randomised, double-blind, placebo-controlled, dose-escalation trial. Ann. Rheum. Dis. 2015;74:1058–1064. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

