The impact of mitotic errors on cell proliferation and tumorigenesis
- PMID: 29802124
- PMCID: PMC6004076
- DOI: 10.1101/gad.314351.118
The impact of mitotic errors on cell proliferation and tumorigenesis
Abstract
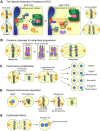
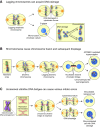
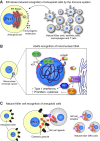
Mitosis is a delicate event that must be executed with high fidelity to ensure genomic stability. Recent work has provided insight into how mitotic errors shape cancer genomes by driving both numerical and structural alterations in chromosomes that contribute to tumor initiation and progression. Here, we review the sources of mitotic errors in human tumors and their effect on cell fitness and transformation. We discuss new findings that suggest that chromosome missegregation can produce a proinflammatory environment and impact tumor responsiveness to immunotherapy. Finally, we survey the vulnerabilities exposed by cell division errors and how they can be exploited therapeutically.
Keywords: aneuploidy; chromosomal instability; mitosis.
© 2018 Levine and Holland; Published by Cold Spring Harbor Laboratory Press.
Figures

References
-
- Artandi SE, Chang S, Lee SL, Alson S, Gottlieb GJ, Chin L, DePinho RA. 2000. Telomere dysfunction promotes non-reciprocal translocations and epithelial cancers in mice. Nature 406: 641–645. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
