Manganese Is Essential for PlcP Metallophosphoesterase Activity Involved in Lipid Remodeling in Abundant Marine Heterotrophic Bacteria
- PMID: 29802183
- PMCID: PMC6052262
- DOI: 10.1128/AEM.01109-18
Manganese Is Essential for PlcP Metallophosphoesterase Activity Involved in Lipid Remodeling in Abundant Marine Heterotrophic Bacteria
Abstract
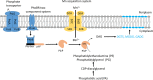
In vast areas of the ocean, microbes must adapt to the availability of scarce nutrients, and a key strategy for reducing the cellular phosphorus (P) quota is to remodel membranes by replacing phospholipids with non-P surrogate lipids. A metallophosphoesterase, PlcP, is essential for lipid remodeling in cosmopolitan marine bacteria of the Roseobacter (e.g., Phaeobacter sp. strain MED193) and SAR11 (e.g., Pelagibacter sp. strain HTCC7211) clades, and transcription of plcP is known to be induced by P limitation. In order to better understand PlcP-mediated lipid remodeling, we sought to characterize PlcP for its metal ion requirement and to determine its selectivity for native bacterial phospholipids. Here, we report the occurrence of a highly conserved binuclear ion center in PlcPs from MED193 and HTCC7211 and show that manganese is the preferred metal for metallophosphoesterase activity. PlcP displayed high activity towards the major bacterial phospholipids, e.g., phosphatidylglycerol but also phosphatidic acid, a key intermediate in phospholipid biosynthesis. In contrast, phosphatidylserine and phosphatidylinositol, both of which are rare lipids in bacteria, are not preferred substrates. These data suggest that PlcP undertakes a generic lipid remodeling role during the cellular response of marine bacteria to P deficiency and that manganese availability may play a key role in regulating the lipid remodeling process.IMPORTANCE Membrane lipids form the structural basis of all cells. In the marine environment, it is well established that phosphorus availability significantly affects lipid composition in cosmopolitan marine bacteria, whereby non-phosphorus-containing lipids are used to replace phospholipids in response to phosphorus stress. Central to this lipid remodeling pathway is a newly identified phospholipase C-type metallophosphoesterase (PlcP). However, little is known about how PlcP activity is regulated. Here, we determined the role of metal ions in regulating PlcP activity and compared PlcP substrate specificities in PlcP enzymes from two model marine bacteria from the marine Roseobacter clade and the SAR11 clade. Our data provide new insights into the regulation of lipid remodeling in these marine bacteria.
Keywords: PlcP; Roseobacter; SAR11; lipid remodeling.
Copyright © 2018 American Society for Microbiology.
Figures





References
-
- Moore CM, Mills MM, Arrigo KR, Berman-Frank I, Bopp L, Boyd PW, Galbraith ED, Geider RJ, Guieu C, Jaccard SL, Jickells TD, La Roche J, Lenton TM, Mahowald NM, Marañón E, Marinov I, Moore JK, Nakatsuka T, Oschlies A, Saito MA, Thingstad TF, Tsudaand A, Ulloa O. 2013. Processes and patterns of oceanic nutrient limitation. Nat Geosci 6:701–710. doi:10.1038/ngeo1765. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

