Enhanced Cardiomyocyte NLRP3 Inflammasome Signaling Promotes Atrial Fibrillation
- PMID: 29802206
- PMCID: PMC6252285
- DOI: 10.1161/CIRCULATIONAHA.118.035202
Enhanced Cardiomyocyte NLRP3 Inflammasome Signaling Promotes Atrial Fibrillation
Erratum in
-
Correction to: Enhanced Cardiomyocyte NLRP3 Inflammasome Signaling Promotes Atrial Fibrillation.Circulation. 2019 Apr 23;139(17):e889. doi: 10.1161/CIR.0000000000000694. Circulation. 2019. PMID: 31013135 No abstract available.
Abstract
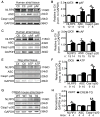
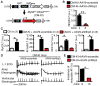
Background: Atrial fibrillation (AF) is frequently associated with enhanced inflammatory response. The NLRP3 (NACHT, LRR, and PYD domain containing protein 3) inflammasome mediates caspase-1 activation and interleukin-1β release in immune cells but is not known to play a role in cardiomyocytes (CMs). Here, we assessed the role of CM NLRP3 inflammasome in AF.
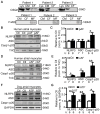
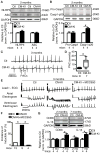
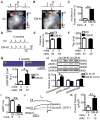
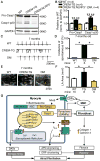
Methods: NLRP3 inflammasome activation was assessed by immunoblot in atrial whole-tissue lysates and CMs from patients with paroxysmal AF or long-standing persistent (chronic) AF. To determine whether CM-specific activation of NLPR3 is sufficient to promote AF, a CM-specific knockin mouse model expressing constitutively active NLRP3 (CM-KI) was established. In vivo electrophysiology was used to assess atrial arrhythmia vulnerability. To evaluate the mechanism of AF, electric activation pattern, Ca2+ spark frequency, atrial effective refractory period, and morphology of atria were evaluated in CM-KI mice and wild-type littermates.
Results: NLRP3 inflammasome activity was increased in the atrial CMs of patients with paroxysmal AF and chronic AF. CM-KI mice developed spontaneous premature atrial contractions and inducible AF, which was attenuated by a specific NLRP3 inflammasome inhibitor, MCC950. CM-KI mice exhibited ectopic activity, abnormal sarcoplasmic reticulum Ca2+ release, atrial effective refractory period shortening, and atrial hypertrophy. Adeno-associated virus subtype-9-mediated CM-specific knockdown of Nlrp3 suppressed AF development in CM-KI mice. Finally, genetic inhibition of Nlrp3 prevented AF development in CREM transgenic mice, a well-characterized mouse model of spontaneous AF.
Conclusions: Our study establishes a novel pathophysiological role for CM NLRP3 inflammasome signaling, with a mechanistic link to the pathogenesis of AF, and establishes the inhibition of NLRP3 as a potential novel AF therapy approach.
Keywords: AAV9; NLRP3 inflammasome; atrial fibrillation; electrical remodeling.
Figures

Comment in
-
Inflammasome activation in AF.Nat Rev Cardiol. 2018 Aug;15(8):442. doi: 10.1038/s41569-018-0045-5. Nat Rev Cardiol. 2018. PMID: 29891969 No abstract available.
-
Inflammation, Inflammasome Activation, and Atrial Fibrillation.Circulation. 2018 Nov 13;138(20):2243-2246. doi: 10.1161/CIRCULATIONAHA.118.036143. Circulation. 2018. PMID: 30571523 Free PMC article. No abstract available.
References
-
- Freeman JV, Wang Y, Akar J, Desai N, Krumholz H. National Trends in Atrial Fibrillation Hospitalization, Readmission, and Mortality for Medicare Beneficiaries, 1999–2013. Circulation. 2017;135:1227–1239. - PubMed
-
- Aviles RJ, Martin DO, Apperson-Hansen C, Houghtaling PL, Rautaharju P, Kronmal RA, Tracy RP, Van Wagoner DR, Psaty BM, Lauer MS, Chung MK. Inflammation as a risk factor for atrial fibrillation. Circulation. 2003;108:3006–3010. - PubMed
-
- Cheng T, Wang XF, Hou YT, Zhang L. Correlation between atrial fibrillation, serum amyloid protein A and other inflammatory cytokines. Mol Med Rep. 2012;6:581–584. - PubMed
-
- Giannopoulos G, Cleman MW, Deftereos S. Inflammation fueling atrial fibrillation substrate: seeking ways to “cool” the heart. Med Chem. 2014;10:663–671. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

