Developmental temperature has persistent, sexually dimorphic effects on zebrafish cardiac anatomy
- PMID: 29802254
- PMCID: PMC5970236
- DOI: 10.1038/s41598-018-25991-8
Developmental temperature has persistent, sexually dimorphic effects on zebrafish cardiac anatomy
Abstract
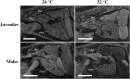
Over the next century, climate change of anthropogenic origin is a major threat to global biodiversity. We show here that developmental temperature can have significant effects on zebrafish cardiac anatomy and swimming performance. Zebrafish embryos were subjected to three developmental temperature treatments (TD = 24, 28 or 32 °C) up to metamorphosis and then all maintained under common conditions (28 °C) to adulthood. We found that developmental temperature affected cardiac anatomy of juveniles and adults even eight months after the different thermal treatments had been applied. The elevation of TD induced a significant increase of the ventricle roundness in juvenile (10% increase) and male (22% increase), but not in female zebrafish. The aerobic exercise performance of adult zebrafish was significantly decreased as TD elevated from 24 to 32 °C. Gene expression analysis that was performed at the end of the temperature treatments revealed significant up-regulation of nppa, myh7 and mybpc3 genes at the colder temperature. Our work provides the first evidence for a direct link between developmental temperature and cardiac form at later life-stages. Our results also add to the emerging rationale for understanding the potential effects of global warming on how fish will perform in their natural environment.
Conflict of interest statement
The authors declare no competing interests.
Figures





References
-
- Poloczanska ES, et al. Global imprint of climate change on marine life. Nat Clim Change. 2013;3:919–925. doi: 10.1038/nclimate1958. - DOI
-
- Heller NE, Zavaleta ES. Biodiversity management in the face of climate change: A review of 22 years of recommendations. Biol Conserv. 2009;142:14–32. doi: 10.1016/j.biocon.2008.10.006. - DOI
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

