Proteomic identification of Axc, a novel beta-lactamase with carbapenemase activity in a meropenem-resistant clinical isolate of Achromobacter xylosoxidans
- PMID: 29802257
- PMCID: PMC5970244
- DOI: 10.1038/s41598-018-26079-z
Proteomic identification of Axc, a novel beta-lactamase with carbapenemase activity in a meropenem-resistant clinical isolate of Achromobacter xylosoxidans
Abstract
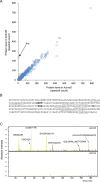
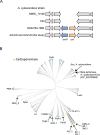
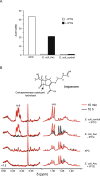
The development of antibiotic resistance during treatment is a threat to patients and their environment. Insight in the mechanisms of resistance development is important for appropriate therapy and infection control. Here, we describe how through the application of mass spectrometry-based proteomics, a novel beta-lactamase Axc was identified as an indicator of acquired carbapenem resistance in a clinical isolate of Achromobacter xylosoxidans. Comparative proteomic analysis of consecutively collected susceptible and resistant isolates from the same patient revealed that high Axc protein levels were only observed in the resistant isolate. Heterologous expression of Axc in Escherichia coli significantly increased the resistance towards carbapenems. Importantly, direct Axc mediated hydrolysis of imipenem was demonstrated using pH shift assays and 1H-NMR, confirming Axc as a legitimate carbapenemase. Whole genome sequencing revealed that the susceptible and resistant isolates were remarkably similar. Together these findings provide a molecular context for the fast development of meropenem resistance in A. xylosoxidans during treatment and demonstrate the use of mass spectrometric techniques in identifying novel resistance determinants.
Conflict of interest statement
The authors declare no competing interests.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

