Effects of Decade Long Freezing Storage on Adipose Derived Stem Cells Functionality
- PMID: 29802353
- PMCID: PMC5970158
- DOI: 10.1038/s41598-018-26546-7
Effects of Decade Long Freezing Storage on Adipose Derived Stem Cells Functionality
Abstract
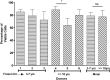
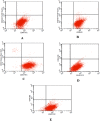
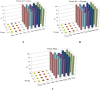
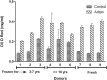
Over the last decade and half, the optimization of cryopreservation for adipose tissue derived stromal/stem cells (ASCs) especially in determining the optimal combination of cryoprotectant type, cooling rate, and thawing rate have been extensively studied. In this study, we examined the functionality of ASCs that have been frozen-stored for more than 10 years denoted as long-term freezing, frozen within the last 3 to 7 years denoted as short-term freezing and compared their response with fresh ASCs. The mean post-thaw viability for long-term frozen group was 78% whereas for short-term frozen group 79% with no significant differences between the two groups. The flow cytometry evaluation of stromal surface markers, CD29, CD90, CD105, CD44, and CD73 indicated the expression (above 95%) in passages P1-P4 in all of the frozen-thawed ASC groups and fresh ASCs whereas the hematopoietic markers CD31, CD34, CD45, and CD146 were expressed extremely low (below 2%) within both the frozen-thawed and fresh cell groups. Quantitative real time polymerase chain reaction (qPCR) analysis revealed some differences between the osteogenic gene expression of long-term frozen group in comparison to fresh ASCs. Intriguingly, one group of cells from the short-term frozen group exhibited remarkably higher expression of osteogenic genes in comparison to fresh ASCs. The adipogenic differentiation potential remained virtually unchanged between all of the frozen-thawed groups and the fresh ASCs. Long-term cryopreservation of ASCs, in general, has a somewhat negative impact on the osteogenic potential of ASCs, especially as it relates to the decrease in osteopontin gene expression but not significantly so with respect to RUNX2 and osteonectin gene expressions. However, the adipogenic potential, post thaw viability, and immunophenotype characteristics remain relatively intact between all the groups.
Conflict of interest statement
During the study period, Drs. X.W. and J.G. were employed at LaCell LLC, a for-profit biotech company cofounded and co-owned by Dr. J.G. and Dr. X.W. The remaining coauthors have no conflicts to disclose.
Figures





References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

