Enhancing the effects of chemotherapy by combined macrophage-mediated photothermal therapy (PTT) and photochemical internalization (PCI)
- PMID: 29802587
- PMCID: PMC6296383
- DOI: 10.1007/s10103-018-2534-5
Enhancing the effects of chemotherapy by combined macrophage-mediated photothermal therapy (PTT) and photochemical internalization (PCI)
Abstract
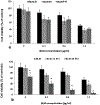
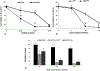
Light-based treatment modalities such as photothermal therapy (PTT) or photochemical internalization (PCI) have been well documented both experimentally and clinically to enhance the efficacy of chemotherapy. The main purpose of this study was to examine the cytotoxic effects of silica-gold nanoshell (AuNS)-loaded macrophage-mediated (MaNS) PTT and bleomycin BLM-PCI on monolayers of squamous cell carcinoma cells. The two modalities were applied separately and in simultaneous combination. Two different wavelengths of light were employed simultaneously, one to activate a highly efficient PCI photosensitizer, AlPcS2a (670 nm) and the other for the MaNS-mediated PTT (810 nm), to evaluate the combined effects of these modalities. The results clearly demonstrated that macrophages could ingest sufficient numbers of silica-gold nanoshells for efficient near infrared (NIR) activated PTT. A significant synergistic effect of simultaneously applied combined PTT and PCI, compared to each modality applied separately, was achieved. Light-driven therapies have the advantage of site specificity, non-invasive and non-toxic application, require inexpensive equipment and can be given as repetitive treatment protocols.
Keywords: Gold-silica nanoshells; Photochemical internalization; Photothermal therapy; Rat macrophages; Squamous cell carcinoma.
Figures



References
-
- Berg K, Selbo PK, Prasmickaite L, Tjelle TE, Sandvig K, Moan J, Gaudernack G, Fodstad O, Kjølsrud S, Anholt H, Rodal GH, Rodal SK, Høgset A (1999) Photochemical internalization: a novel technology for delivery of macromolecules into cytosol. Cancer Res 59(6):1180–1183 - PubMed
-
- Berg K, Folini M, Prasmickaite L, Selbo PK, Bonsted A, Engesaeter BØ, Zaffaroni N, Weyergang A, Dietze A, Maelandsmo GM, Wagner E, Norum OJ, Høgset A (2007) Photochemical internalization: a new tool for drug delivery. Curr Pharm Biotechnol 8(6):362–372 - PubMed
-
- Norum OJ, Giercksky KE, Berg K (2009) Photochemical internalization as an adjunct to marginal surgery in a human sarcoma model. Photochem Photobiol Sci 8(6):758–762 - PubMed
-
- Selbo PK, Weyergang A, Høgset A, Norum OJ, Berstad MB, Vikdal M, Berg K (2010) Photochemical internalization provides time- and space-controlled endolysosomal escape of therapeutic molecules. J Control Release 148(1):2–12 - PubMed
-
- Weyergang A, Berstad ME, Bull-Hansen B, Olsen CE, Selbo PK, Berg K (2010) 2015 photochemical activation of drugs for the treatment of therapy-resistant cancers. Photochem Photobiol Sci 14:1465 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

