Drugs in Development for Malaria
- PMID: 29802605
- PMCID: PMC6013505
- DOI: 10.1007/s40265-018-0911-9
Drugs in Development for Malaria
Abstract
The last two decades have seen a surge in antimalarial drug development with product development partnerships taking a leading role. Resistance of Plasmodium falciparum to the artemisinin derivatives, piperaquine and mefloquine in Southeast Asia means new antimalarials are needed with some urgency. There are at least 13 agents in clinical development. Most of these are blood schizonticides for the treatment of uncomplicated falciparum malaria, under evaluation either singly or as part of two-drug combinations. Leading candidates progressing through the pipeline are artefenomel-ferroquine and lumefantrine-KAF156, both in Phase 2b. Treatment of severe malaria continues to rely on two parenteral drugs with ancient forebears: artesunate and quinine, with sevuparin being evaluated as an adjuvant therapy. Tafenoquine is under review by stringent regulatory authorities for approval as a single-dose treatment for Plasmodium vivax relapse prevention. This represents an advance over standard 14-day primaquine regimens; however, the risk of acute haemolytic anaemia in patients with glucose-6-phosphate dehydrogenase deficiency remains. For disease prevention, several of the newer agents show potential but are unlikely to be recommended for use in the main target groups of pregnant women and young children for some years. Latest predictions are that the malaria burden will continue to be high in the coming decades. This fact, coupled with the repeated loss of antimalarials to resistance, indicates that new antimalarials will be needed for years to come. Failure of the artemisinin-based combinations in Southeast Asia has stimulated a reappraisal of current approaches to combination therapy for malaria with incorporation of three or more drugs in a single treatment under consideration.
Conflict of interest statement
APP was an investigator on trials of artefenomel sponsored by Medicines for Malaria Venture and KAF156 and cipargamin sponsored by Novartis. EAA was a co-investigator on studies of DHA–piperaquine funded by Medicines for Malaria Venture and of artesunate–mefloquine funded by Drugs for Neglected Disease Initiative.
Figures
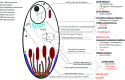
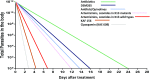

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

