The microbiota of traumatic, open fracture wounds is associated with mechanism of injury
- PMID: 29802752
- PMCID: PMC6202213
- DOI: 10.1111/wrr.12642
The microbiota of traumatic, open fracture wounds is associated with mechanism of injury
Abstract
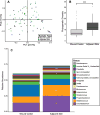
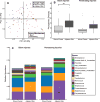
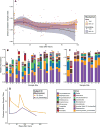
Open fractures are characterized by disruption of the skin and soft tissue, which allows for microbial contamination and colonization. Preventing infection-related complications of open fractures and other acute wounds remains an evolving challenge due to an incomplete understanding of how microbial colonization and contamination influence healing and outcomes. Culture-independent molecular methods are now widely used to study human-associated microbial communities without introducing culture biases. Using such approaches, the objectives of this study were to (1) define the long-term temporal microbial community dynamics of open fracture wounds and (2) examine microbial community dynamics with respect to clinical and demographic factors. Fifty-two subjects with traumatic open fracture wounds (32 blunt and 20 penetrating injuries) were enrolled prospectively and sampled longitudinally from presentation to the emergency department (ED) and at each subsequent inpatient or outpatient encounter. Specimens were collected from both the wound center and adjacent skin. Culture-independent sequencing of the 16S ribosomal RNA gene was employed to identify and characterize microbiota. Upon presentation to the ED and time points immediately following, sample collection site (wound or adjacent skin) was the most defining feature discriminating microbial profiles. Microbial composition of adjacent skin and wound center converged over time. Mechanism of injury most strongly defined the microbiota after initial convergence. Further analysis controlling for race, gender, and age revealed that mechanism of injury remained a significant discriminating feature throughout the continuum of care. We conclude that the microbial communities associated with open fracture wounds are dynamic in nature until eventual convergence with the adjacent skin community during healing, with mechanism of injury as an important feature affecting both diversity and composition of the microbiota. A more complete understanding of the factors influencing microbial contamination and/or colonization in open fractures is a critical foundation for identifying markers indicative of outcome and deciphering their respective contributions to healing and/or complication.
© 2018 by the Wound Healing Society.
Conflict of interest statement
Figures

References
-
- Harley BJ, Beaupre LA, Jones CA, Dulai SK, Weber DW. The effect of time to definitive treatment on the rate of nonunion and infection in open fractures. J Orthop Trauma. 2002;16(7):484–90. - PubMed
-
- Rajilić‐Stojanović M, Smidt H, De Vos WM. Diversity of the human gastrointestinal tract microbiota revisited. Environ Microbiol. 2007;9(9):2125–36. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- NIAMS/NIH T32AR007465/University of Pennsylvania Department of Dermatology/International
- R01 AR066663/AR/NIAMS NIH HHS/United States
- NINR R01N R015639/NH/NIH HHS/United States
- NIAMS R00AR060873/NH/NIH HHS/United States
- T32AR007465/AR/NIAMS NIH HHS/United States
- R01NR015639/NR/NINR NIH HHS/United States
- R00AR060873/AR/NIAMS NIH HHS/United States
- 029/Orthopaedic Trauma Association/International
- R01 NR015639/NR/NINR NIH HHS/United States
- T32 AR007465/AR/NIAMS NIH HHS/United States
- #029 (SM)/Orthopaedic Trauma Association/International
- R01AR066663/AR/NIAMS NIH HHS/United States
- US Department of Defense National Defense Science and Engineering Graduate Fellowship/International
- R00 AR060873/AR/NIAMS NIH HHS/United States
- NIAMS R01AR066663/NH/NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources

