Administration of nicotinamide riboside prevents oxidative stress and organ injury in sepsis
- PMID: 29803807
- PMCID: PMC6236680
- DOI: 10.1016/j.freeradbiomed.2018.05.073
Administration of nicotinamide riboside prevents oxidative stress and organ injury in sepsis
Abstract
Aims: Sepsis-caused multiple organ failure remains the major cause of morbidity and mortality in intensive care units. Nicotinamide riboside (NR) is a precursor of nicotinamide adenine dinucleotide (NAD+), which is important in regulating oxidative stress. This study investigated whether administration of NR prevented oxidative stress and organ injury in sepsis.
Methods: Mouse sepsis models were induced by injection of lipopolysaccharides (LPS) or feces-injection-in-peritoneum. NR was given before sepsis onset. Cultured macrophages and endothelial cells were incubated with various agents.
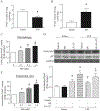
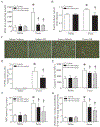
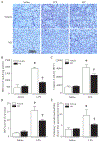
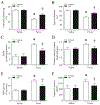
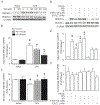
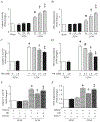
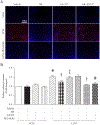
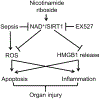
Results: Administration of NR elevated the NAD+ levels, and elicited a reduction of oxidative stress, inflammation and caspase-3 activity in lung and heart tissues, which correlated with attenuation of pulmonary microvascular permeability and myocardial dysfunction, leading to less mortality in sepsis models. These protective effects of NR were associated with decreased levels of plasma high mobility group box-1 (HMGB1) in septic mice. Consistently, pre-treatment of macrophages with NR increased NAD+ content and reduced HMGB1 release upon LPS stimulation. NR also prevented reactive oxygen species (ROS) production and apoptosis in endothelial cells induced by a conditioned-medium collected from LPS-treated macrophages. Furthermore, inhibition of SIRT1 by EX527 offset the negative effects of NR on HMGB1 release in macrophages, and ROS and apoptosis in endothelial cells.
Conclusions: Administration of NR prevents lung and heart injury, and improves the survival in sepsis, likely by inhibiting HMGB1 release and oxidative stress via the NAD+/SIRT1 signaling. Given NR has been used as a health supplement, it may be a useful agent to prevent organ injury in sepsis.
Keywords: HMGB1; Nicotinamide riboside; Oxidative stress; SIRT1; Sepsis.
Copyright © 2018 Elsevier Inc. All rights reserved.
Figures

References
-
- Martin CM, Priestap F, Fisher H, Fowler RA, Heyland DK, Keenan SP, Longo CJ, Morrison T, Bentley D, Antman N, A prospective, observational registry of patients with severe sepsis: the Canadian sepsis treatment and response registry, Crit. Care Med 37 (1) (2009) 81–88. - PubMed
-
- Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche JD, Coopersmith CM, Hotchkiss RS, Levy MM, Marshall JC, Martin GS, Opal SM, Rubenfeld GD, van der Poll T, Vincent JL, Angus DC, The third international consensus definitions for sepsis and septic shock (Sepsis-3), JAMA 315 (8) (2016) 801–810. - PMC - PubMed
-
- Howell MD, Davis AM, Management of sepsis and septic shock, JAMA 317 (8) (2017) 847–848. - PubMed
-
- Wheeler AP, Bernard GR, Acute lung injury and the acute respiratory distress syndrome: a clinical review, Lancet 369 (9572) (2007) 1553–1564. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

