Intestinal HIF-1α deletion exacerbates alcoholic liver disease by inducing intestinal dysbiosis and barrier dysfunction
- PMID: 29803899
- PMCID: PMC6615474
- DOI: 10.1016/j.jhep.2018.05.021
Intestinal HIF-1α deletion exacerbates alcoholic liver disease by inducing intestinal dysbiosis and barrier dysfunction
Abstract
Background & aims: Alcoholic liver disease (ALD) is characterized by gut dysbiosis and increased gut permeability. Hypoxia inducible factor 1α (HIF-1α) has been implicated in transcriptional regulation of intestinal barrier integrity and inflammation. We aimed to test the hypothesis that HIF-1α plays a critical role in gut microbiota homeostasis and the maintenance of intestinal barrier integrity in a mouse model of ALD.
Methods: Wild-type (WT) and intestinal epithelial-specific Hif1a knockout mice (IEhif1α-/-) were pair-fed modified Lieber-DeCarli liquid diet containing 5% (w/v) alcohol or isocaloric maltose dextrin for 24 days. Serum levels of alanine aminotransferase and endotoxin were determined. Fecal microbiota were assessed. Liver steatosis and injury, and intestinal barrier integrity were evaluated.
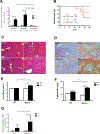
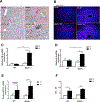
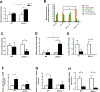
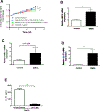
Results: Alcohol feeding increased serum levels of alanine aminotransferase and lipopolysaccharide, hepatic triglyceride concentration, and liver injury in the WT mice. These deleterious effects were exaggerated in IEhif1α-/- mice. Alcohol exposure resulted in greater reduction of the expression of intestinal epithelial tight junction proteins, claudin-1 and occludin, in IEhif1α-/- mice. In addition, cathelicidin-related antimicrobial peptide and intestinal trefoil factor were further decreased by alcohol in IEhif1α-/- mice. Metagenomic analysis showed increased gut dysbiosis and significantly decreased Firmicutes/Bacteroidetes ratio in IEhif1α-/- mice compared to the WT mice exposed to alcohol. An increased abundance of Akkermansia and a decreased level of Lactobacillus in IEhif1α-/- mice were also observed. Non-absorbable antibiotic treatment reversed the liver steatosis in both WT and IEhif1α-/- mice.
Conclusion: Intestinal HIF-1α is essential for the adaptative response to alcohol-induced changes in intestinal microbiota and barrier function associated with elevated endotoxemia and hepatic steatosis and injury.
Lay summary: Alcohol consumption alters gut microbiota and multiple intestinal barrier protecting factors that are regulated by intestinal hypoxia-inducible factor 1α (HIF-1α). Absence of intestinal HIF-1α exacerbates gut leakiness leading to an increased translocation of bacteria and bacterial products to the liver, consequently causing alcoholic liver disease. Intestinal specific upregulation of HIF-1α could be developed as a novel approach for the treatment of alcoholic liver disease.
Keywords: Alcoholic Liver Disease; Gut Microbiota; Gut-Liver axis; Hypoxia-Inducible Factor-1α; Intestinal Barrier.
Copyright © 2018 European Association for the Study of the Liver. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Figures



Comment in
-
The multi-dimensional role of intestinal HIFs in liver pathobiology.J Hepatol. 2018 Oct;69(4):772-773. doi: 10.1016/j.jhep.2018.07.012. Epub 2018 Aug 10. J Hepatol. 2018. PMID: 30104025 No abstract available.
References
-
- Adachi Y, Moore LE, Bradford BU, Gao W, Thurman RG. Antibiotics prevent liver injury in rats following long-term exposure to ethanol. Gastroenterology 1995;108:218–224. - PubMed
-
- Parlesak A, Schafer C, Schutz T, Bode JC, Bode C. Increased intestinal permeability to macromolecules and endotoxemia in patients with chronic alcohol abuse in different stages of alcohol-induced liver disease. J Hepatol 2000;32:742–747. - PubMed
MeSH terms
Substances
Grants and funding
- S10 OD020106/OD/NIH HHS/United States
- R01 AA024405/AA/NIAAA NIH HHS/United States
- P20 GM113226/GM/NIGMS NIH HHS/United States
- R21 AA020848/AA/NIAAA NIH HHS/United States
- P50 AA024337/AA/NIAAA NIH HHS/United States
- R01 AA021434/AA/NIAAA NIH HHS/United States
- R01 AA023681/AA/NIAAA NIH HHS/United States
- U01 AA022489/AA/NIAAA NIH HHS/United States
- I01 BX002996/BX/BLRD VA/United States
- R01 AA020265/AA/NIAAA NIH HHS/United States
- U01 AA021901/AA/NIAAA NIH HHS/United States
- R01 AA023190/AA/NIAAA NIH HHS/United States
- R21 AA022416/AA/NIAAA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources

