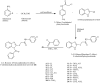
Synthesis and evaluation of antimicrobial, antitubercular and anticancer activities of 2-(1-benzoyl-1H-benzo[d]imidazol-2-ylthio)-N-substituted acetamides
- PMID: 29804151
- PMCID: PMC5971037
- DOI: 10.1186/s13065-018-0432-3
Synthesis and evaluation of antimicrobial, antitubercular and anticancer activities of 2-(1-benzoyl-1H-benzo[d]imidazol-2-ylthio)-N-substituted acetamides
Abstract
Background: The study describes the synthesis, characterization, in vitro antimicrobial and anticancer evaluation of a series of 2-(1-benzoyl-1H-benzo[d]imidazol-2-ylthio)-N-substituted acetamide derivatives. The synthesized derivatives were also assessed for in vitro antitubercular activity against Mycobacterium tuberculosis H37Rv. The compounds found active in in vitro study were assessed for their in vivo antitubercular activity in mice models and for their inhibitory action on vital mycobacterial enzymes viz, isocitrate lyase, pantothenate synthetase and chorismate mutase.
Results: Compounds 8, 9 and 11 emerged out as excellent antimicrobial agents in antimicrobial assays when compared to standard antibacterial and antifungal drugs. The results of anticancer activity displayed that majority of the derivatives were less cytotoxic than standard drugs (tamoxifen and 5-fluorouracil) towards MCF7 and HCT116 cell lines. However, compound 2 (IC50 = 0.0047 µM/ml) and compound 10 (IC50 = 0.0058 µM/ml) showed highest cytotoxicity against MCF7 and HCT116 cell lines, respectively. The results of in vivo antitubercular activity revealed that a dose of 1.34 mg/kg was found to be safe for the synthesized compounds. The toxic dose of the compounds was 5.67 mg/kg while lethal dose varied from 1.81 to 3.17 mg/kg body weight of the mice. Compound 18 inhibited all the three mycobacterial enzymes to the highest level in comparison to the other synthesized derivatives but showed lesser inhibition as compared to streptomycin sulphate.
Conclusions: A further research on most active synthesized compounds as lead molecules may result in discovery of novel anticancer and antitubercular agents.
Keywords: Cytotoxic; In vitro; Isocitrate lyase; MCF7, HCT116; Pantothenate synthetase; Resistance.
Figures
References
-
- Singh LR, Avula SR, Raj S, Srivastava A, Palnati GR, Tripathi CKM, Pasupuleti M, Sashidhara KV. Coumarin–benzimidazole hybrids as a potent antimicrobial agent: synthesis and biological elevation. J Antibiot. 2017 - PubMed
-
- WHO annual TB report 2017. http://apps.who.int/iris/bitstream/10665/254762/1/978929022584-eng.pdf. Accessed 24 July 2017
LinkOut - more resources
Full Text Sources
Other Literature Sources