A phase II study of frontline paclitaxel/carboplatin/bevacizumab, paclitaxel/carboplatin/temsirolimus, or ixabepilone/carboplatin/bevacizumab in advanced/recurrent endometrial cancer
- PMID: 29804638
- PMCID: PMC6179372
- DOI: 10.1016/j.ygyno.2018.05.018
A phase II study of frontline paclitaxel/carboplatin/bevacizumab, paclitaxel/carboplatin/temsirolimus, or ixabepilone/carboplatin/bevacizumab in advanced/recurrent endometrial cancer
Abstract
Objective: Paclitaxel and carboplatin (PC) is a standard initial therapy for advanced endometrial cancer. We evaluated the efficacy and tolerability of incorporating three novel agents into initial therapy.
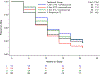
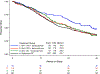
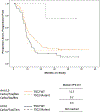
Methods: In this randomized phase II trial, patients with chemotherapy-naïve stage III/IVA (with measurable disease) and stage IVB or recurrent (with or without measurable disease) endometrial cancer were randomly assigned to treatment with PC plus bevacizumab (Arm 1), PC plus temsirolimus (Arm 2) or ixabepilone and carboplatin (IC) plus bevacizumab (Arm 3). The primary endpoint was progression-free survival (PFS). Comparable patients on the PC Arm of trial GOG209 were used as historical controls. Secondary endpoints were response rate, overall survival (OS), and safety.
Results: Overall, 349 patients were randomized. PFS duration was not significantly increased in any experimental arm compared with historical controls (p > 0.039). Treatment HRs (92% CI) for Arms 1, 2, and 3 relative to controls were 0.81 (0.63-1.02), 1.22 (0.96-1.55) and 0.87 (0.68-1.11), respectively. Response rates were similar across arms (60%, 55% and 53%, respectively). Relative to controls, OS duration (with censoring at 36 months), was significantly increased in Arm 1 (p < 0.039) but not in Arms 2 and 3; the HRs (92% CIs) were 0.71 (0.55-0.91), 0.99 (0.78-1.26), and 0.97 (0.77-1.23), respectively. No new safety signals were identified. Common mutations and rates of mismatch repair protein loss are described by histotype. Potential predictive biomarkers for temsirolimus and bevacizumab were identified.
Conclusion: PFS was not significantly increased in any experimental arm compared to historical controls. NRG Oncology/Gynecologic Oncology Group Study GOG-86P.
Keywords: Bevacizumab; Carboplatin; Endometrial cancer; Ixabepilone; Paclitaxel; Temsirolimus.
Copyright © 2018 Elsevier Inc. All rights reserved.
Conflict of interest statement
CONFLICT OF INTEREST STATEMENT
Outside the submitted work, C. Aghajanian has participated on the advisory boards of Tesaro, Clovis, Cerulean, Bayer, and VentiRx; she has also participated on the steering committee of Mateon Therapeutics. Outside the submitted work, M. A. Powell has received consultancy fees from Roche/Genentech, AstraZeneca, Clovis Oncology, Tesaro, and Merck; he has also received payments for lectures (e.g., speaker’s bureau) from Roche/Genentech, AstraZeneca, Clovis Oncology, Tesaro, and Merck; he has also received royalties from Up to Date. For the work under consideration, A. Alvarez Secord has received grant funding from NRG Oncology/Legacy GOG; outside the submitted work, she has also received consultancy fees from Alexion, Astex, AstraZeneca, Boerhinger Ingelheim, GlaxoSmithKline, Clovis, Janssen/Johnson & Johnson, Myriad, Roche/Genentech, and Tesaro; her institution has also received grants/pending grants from AbbVie, Amgen, Astellas Pharma Inc., Astex Pharmaceuticals Inc., AstraZeneca, Boerhinger Ingelheim, Bristol Myers Squibb, Eisai, Endocyte, Exelixis, Incyte, Merck, PharmaMar, Prima Biomed, Roche/Genentech, and Tesaro. Outside the submitted work, K. S. Tewari has participated in a speaker’s bureau for Roche. For the work under consideration, D. M. O’Malley’s institution has received grant funding from NRG Oncology/GOG; outside the submitted work, he has received consultancy fees (e.g., advisory board, steering committee) from Clovis, Tesaro, AstraZeneca, Abbvie, Immunogen, GOG, Myriad, and Amgen; outside the submitted work, his institution has received funding for clinical trials from Clovis, AstraZeneca, Serono, Iovance Biotherapeutics, Inc., GOG, Tesaro, Ludwig Institute for Cancer Research Ltd, inVentiv Health Clinical, ImmunoGen, Inc., Stemcentrx, Inc., Agenus, Inc., Bristol-Myers Squibb, Array BioPharma, Inc., TRACON Pharmaceuticals, Janssen Research and Development, LLC, PRA Intl, Ajinomoto Co, Inc., INC Research, Inc., and ERGOMED Clinical Research Ltd. Outside the submitted work, R. A. Soslow has received royalties from Cambridge Press and Springer Publishing. Outside the submitted work, K. Moore has participated on the advisory boards of Clovis, Astra Zeneca, Tesaro, Immunogen, Genentech/Roche, VBL Therapeutics, and Merck.
Figures
References
-
- Miller D, Filiaci V, Fleming G, et al. Randomized phase III noninferiority trial of first line chemotherapy for metastatic or recurrent endometrial carcinoma: a Gynecologic Oncology Group study. Gynecol Oncol 125: 771–773, 2012. (Abstract)
-
- Alvarez EA, Brady WE, Walker JL, et al. Phase II trial of combination bevacizumab and temsirolimus in the treatment of recurrent or persistent endometrial carcinoma: a Gynecologic Oncology Group study. Gynecol Oncol 129(1):22–27, 2013. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources