Measuring mitotic forces
- PMID: 29804669
- PMCID: PMC7286078
- DOI: 10.1016/bs.mcb.2018.03.007
Measuring mitotic forces
Abstract
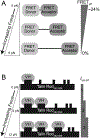
Productive chromosome movements require that a large multiprotein complex called the kinetochore assemble on sister centromeres. The kinetochore fulfills two critical functions as (1) the physical linkage between chromosomes and spindle microtubules and (2) a mechanomolecular sensor that relays a spindle assembly checkpoint signal delaying anaphase onset until chromosomes are attached to spindle microtubules and bioriented. Given its central roles in such a vital process, the kinetochore is one of the most important force-transducing structures in cells; yet it has been technically challenging to measure kinetochore forces. Barriers to measuring cellular forces have begun to be broken by the development of fluorescence-based tension sensors. In this chapter, two methods will be described for measuring kinetochore forces in living cells and strategies for applying these sensors to other force-transducing processes and molecules will be discussed.
Keywords: Force; Kinetochore; Meiosis; Microtubules; Mitosis; Tension.
© 2018 Elsevier Inc. All rights reserved.
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

