Implications of Centers for Medicare & Medicaid Services Severe Sepsis and Septic Shock Early Management Bundle and Initial Lactate Measurement on the Management of Sepsis
- PMID: 29804795
- PMCID: PMC6113629
- DOI: 10.1016/j.chest.2018.03.025
Implications of Centers for Medicare & Medicaid Services Severe Sepsis and Septic Shock Early Management Bundle and Initial Lactate Measurement on the Management of Sepsis
Abstract
Background: Sepsis remains a significant cause of morbidity and mortality in the United States, leading to the implementation of the Severe Sepsis and Septic Shock Early Management Bundle (SEP-1). SEP-1 identifies patients with "severe sepsis" via clinical and laboratory criteria and mandates interventions, including lactate draws and antibiotics, within a specific time window. We sought to characterize the patients affected and to study the implications of SEP-1 on patient care and outcomes.
Methods: All adults admitted to the University of Chicago from November 2008 to January 2016 were eligible. Modified SEP-1 criteria were used to identify appropriate patients. Time to lactate draw and antibiotic and IV fluid administration were calculated. In-hospital mortality was examined.
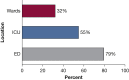
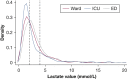
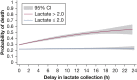
Results: Lactates were measured within the mandated window 32% of the time on the ward (n = 505) compared with 55% (n = 818) in the ICU and 79% (n = 2,144) in the ED. Patients with delayed lactate measurements demonstrated the highest in-hospital mortality at 29%, with increased time to antibiotic administration (median time, 3.9 vs 2.0 h). Patients with initial lactates > 2.0 mmol/L demonstrated an increase in the odds of death with hourly delay in lactate measurement (OR, 1.02; 95% CI, 1.0003-1.05; P = .04).
Conclusions: Delays in lactate measurement are associated with delayed antibiotics and increased mortality in patients with initial intermediate or elevated lactate levels. Systematic early lactate measurement for all patients with sepsis will lead to a significant increase in lactate draws that may prompt more rapid physician intervention for patients with abnormal initial values.
Keywords: critical care; lactic acid; sepsis; septic shock.
Copyright © 2018 American College of Chest Physicians. Published by Elsevier Inc. All rights reserved.
Figures
Comment in
-
Lactate Measurements: A Guide to Therapy or to Quality?Chest. 2018 Dec;154(6):1461. doi: 10.1016/j.chest.2018.07.041. Chest. 2018. PMID: 30526976 No abstract available.
-
Response.Chest. 2018 Dec;154(6):1462. doi: 10.1016/j.chest.2018.08.1025. Chest. 2018. PMID: 30526977 No abstract available.
Similar articles
-
Association Between Implementation of the Severe Sepsis and Septic Shock Early Management Bundle Performance Measure and Outcomes in Patients With Suspected Sepsis in US Hospitals.JAMA Netw Open. 2021 Dec 1;4(12):e2138596. doi: 10.1001/jamanetworkopen.2021.38596. JAMA Netw Open. 2021. PMID: 34928358 Free PMC article.
-
Lactate measurements in sepsis-induced tissue hypoperfusion: results from the Surviving Sepsis Campaign database.Crit Care Med. 2015 Mar;43(3):567-73. doi: 10.1097/CCM.0000000000000742. Crit Care Med. 2015. PMID: 25479113
-
A combination of early warning score and lactate to predict intensive care unit transfer of inpatients with severe sepsis/septic shock.Korean J Intern Med. 2015 Jul;30(4):471-7. doi: 10.3904/kjim.2015.30.4.471. Epub 2015 Jun 29. Korean J Intern Med. 2015. PMID: 26161013 Free PMC article.
-
Evidence Underpinning the Centers for Medicare & Medicaid Services' Severe Sepsis and Septic Shock Management Bundle (SEP-1): A Systematic Review.Ann Intern Med. 2018 Apr 17;168(8):558-568. doi: 10.7326/M17-2947. Epub 2018 Feb 20. Ann Intern Med. 2018. PMID: 29459977 Free PMC article.
-
Antibiotic- and Fluid-Focused Bundles Potentially Improve Sepsis Management, but High-Quality Evidence Is Lacking for the Specificity Required in the Centers for Medicare and Medicaid Service's Sepsis Bundle (SEP-1).Crit Care Med. 2019 Oct;47(10):1290-1300. doi: 10.1097/CCM.0000000000003892. Crit Care Med. 2019. PMID: 31369426 Free PMC article.
Cited by
-
National Performance on the Medicare SEP-1 Sepsis Quality Measure.Crit Care Med. 2019 Aug;47(8):1026-1032. doi: 10.1097/CCM.0000000000003613. Crit Care Med. 2019. PMID: 30585827 Free PMC article.
-
Angiotensinogen: a new era beyond lactate as a biomarker?Crit Care. 2024 Dec 2;28(1):398. doi: 10.1186/s13054-024-05164-y. Crit Care. 2024. PMID: 39623475 Free PMC article. No abstract available.
-
Impact on outcomes of measuring lactates prior to ICU in unselected heterogeneous critically ill patients: A propensity score analysis.PLoS One. 2022 Nov 28;17(11):e0277948. doi: 10.1371/journal.pone.0277948. eCollection 2022. PLoS One. 2022. PMID: 36441770 Free PMC article.
-
Modulation of oxidative and nitrosative stress attenuates microvascular hyperpermeability in ovine model of Pseudomonas aeruginosa sepsis.Sci Rep. 2021 Dec 14;11(1):23966. doi: 10.1038/s41598-021-03320-w. Sci Rep. 2021. PMID: 34907252 Free PMC article.
-
Potential biomarkers in septic shock besides lactate.Exp Biol Med (Maywood). 2020 Jun;245(12):1066-1072. doi: 10.1177/1535370220919076. Epub 2020 Apr 10. Exp Biol Med (Maywood). 2020. PMID: 32276542 Free PMC article. Review.
References
-
- Liu V., Escobar G.J., Green J.D. Hospital deaths in patients with sepsis from 2 independent cohorts. JAMA. 2014;312:90–92. - PubMed
-
- Martin G.S., Mannino D.M., Eaton S., Moss M. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med. 2003;348(16):1546–1554. - PubMed
-
- Angus D.C., Line-Zwirble W.T., Lidicker J., Clermont G., Carcillo J., Pinsky M.R. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29(7):1303–1310. - PubMed
-
- Dellinger R.P., Levy M.M., Rhodes A., Surviving Sepsis Campaign Guidelines Committee including the Pediatric Subgroup Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med. 2013;41(2):580–637. - PubMed
-
- Specifications Manual for National Hospital Inpatient Quality Measures Discharges 10-01-15 (4Q15) through 06-30-16 (2Q16). Specifications manual for National Inpatient Quality Measures. The Joint Commission. 2016.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

