Distant Insulin Signaling Regulates Vertebrate Pigmentation through the Sheddase Bace2
- PMID: 29804876
- PMCID: PMC5991976
- DOI: 10.1016/j.devcel.2018.04.025
Distant Insulin Signaling Regulates Vertebrate Pigmentation through the Sheddase Bace2
Abstract
Patterning of vertebrate melanophores is essential for mate selection and protection from UV-induced damage. Patterning can be influenced by circulating long-range factors, such as hormones, but it is unclear how their activity is controlled in recipient cells to prevent excesses in cell number and migration. The zebrafish wanderlust mutant harbors a mutation in the sheddase bace2 and exhibits hyperdendritic and hyperproliferative melanophores that localize to aberrant sites. We performed a chemical screen to identify suppressors of the wanderlust phenotype and found that inhibition of insulin/PI3Kγ/mTOR signaling rescues the defect. In normal physiology, Bace2 cleaves the insulin receptor, whereas its loss results in hyperactive insulin/PI3K/mTOR signaling. Insulin B, an isoform enriched in the head, drives the melanophore defect. These results suggest that insulin signaling is negatively regulated by melanophore-specific expression of a sheddase, highlighting how long-distance factors can be regulated in a cell-type-specific manner.
Keywords: PI3K; bace2; insulin; insulin receptor; mTOR; melanocyte; melanophore; pigment patterning; zebrafish.
Published by Elsevier Inc.
Conflict of interest statement
The authors declare no competing interests.
Figures



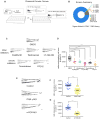
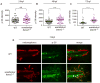
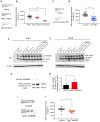

Comment in
-
Melanophores Tune Out the Noise to Make Stripes.Dev Cell. 2018 Jun 4;45(5):544-545. doi: 10.1016/j.devcel.2018.05.014. Dev Cell. 2018. PMID: 29870715
References
-
- Baynash AG, Hosoda K, Giaid A, Richardson JA, Emoto N, Hammer RE, Yanagisawa M. Interaction of endothelin-3 with endothelin-B receptor is essential for development of epidermal melanocytes and enteric neurons. Cell. 1994;79:1277–1285. - PubMed
-
- van Bebber F, Hruscha A, Willem M, Schmid B, Haass C. Loss of Bace2 in zebrafish affects melanocyte migration and is distinct from Bace1 knock out phenotypes. J Neurochem. 2013;127:471–481. - PubMed
-
- Bravo DA, Gleason JB, Sanchez RI, Roth RA, Fuller RS. Accurate and efficient cleavage of the human insulin proreceptor by the human proprotein-processing protease furin. Characterization and kinetic parameters using the purified, secreted soluble protease expressed by a recombinant baculovirus. J Biol Chem. 1994;269:25830–25837. - PubMed
-
- Brou C, Logeat F, Gupta N, Bessia C, LeBail O, Doedens JR, Cumano A, Roux P, Black RA, Israël A. A novel proteolytic cleavage involved in Notch signaling: the role of the disintegrin-metalloprotease TACE. Mol Cell. 2000;5:207–216. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

