An ERK-Dependent Feedback Mechanism Prevents Hematopoietic Stem Cell Exhaustion
- PMID: 29804890
- PMCID: PMC5988582
- DOI: 10.1016/j.stem.2018.05.003
An ERK-Dependent Feedback Mechanism Prevents Hematopoietic Stem Cell Exhaustion
Abstract
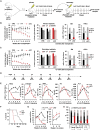
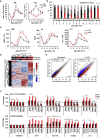
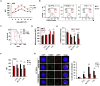
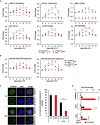
Hematopoietic stem cells (HSCs) sustain hematopoiesis throughout life. HSCs exit dormancy to restore hemostasis in response to stressful events, such as acute blood loss, and must return to a quiescent state to prevent their exhaustion and resulting bone marrow failure. HSC activation is driven in part through the phosphatidylinositol 3-kinase (PI3K)/AKT/mTORC1 signaling pathway, but less is known about the cell-intrinsic pathways that control HSC dormancy. Here, we delineate an ERK-dependent, rate-limiting feedback mechanism that controls HSC fitness and their re-entry into quiescence. We show that the MEK/ERK and PI3K pathways are synchronously activated in HSCs during emergency hematopoiesis and that feedback phosphorylation of MEK1 by activated ERK counterbalances AKT/mTORC1 activation. Genetic or chemical ablation of this feedback loop tilts the balance between HSC dormancy and activation, increasing differentiated cell output and accelerating HSC exhaustion. These results suggest that MEK inhibitors developed for cancer therapy may find additional utility in controlling HSC activation.
Keywords: AKT/mTORC1 pathway; ERK pathway; emergency hematopoiesis; feedback regulation of signaling; hematopoietic stem cell activation; hematopoietic stem cell exhaustion; hematopoietic stem cells; intracellular signaling; mitochondrial fitness; oxidative stress.
Copyright © 2018 The Author(s). Published by Elsevier Inc. All rights reserved.
Figures



Similar articles
-
Protection of hematopoietic stem cells from stress-induced exhaustion and aging.Curr Opin Hematol. 2020 Jul;27(4):225-231. doi: 10.1097/MOH.0000000000000586. Curr Opin Hematol. 2020. PMID: 32398455 Review.
-
Activation of the MAP kinase pathway by c-Kit is PI-3 kinase dependent in hematopoietic progenitor/stem cell lines.Blood. 2004 Jul 1;104(1):51-7. doi: 10.1182/blood-2003-07-2554. Epub 2004 Mar 2. Blood. 2004. PMID: 14996702
-
MEK1 is required for PTEN membrane recruitment, AKT regulation, and the maintenance of peripheral tolerance.Mol Cell. 2013 Apr 11;50(1):43-55. doi: 10.1016/j.molcel.2013.01.037. Epub 2013 Feb 28. Mol Cell. 2013. PMID: 23453810 Free PMC article.
-
Adiponectin Enhances Quiescence Exit of Murine Hematopoietic Stem Cells and Hematopoietic Recovery Through mTORC1 Potentiation.Stem Cells. 2017 Jul;35(7):1835-1848. doi: 10.1002/stem.2640. Epub 2017 May 23. Stem Cells. 2017. PMID: 28480607
-
Molecular regulation of hematopoietic stem cell quiescence.Cell Mol Life Sci. 2022 Mar 31;79(4):218. doi: 10.1007/s00018-022-04200-w. Cell Mol Life Sci. 2022. PMID: 35357574 Free PMC article. Review.
Cited by
-
Hematopoietic Stem Cell Metabolism during Development and Aging.Dev Cell. 2020 Jul 20;54(2):239-255. doi: 10.1016/j.devcel.2020.06.029. Dev Cell. 2020. PMID: 32693057 Free PMC article. Review.
-
tRNA m1A modification regulate HSC maintenance and self-renewal via mTORC1 signaling.Nat Commun. 2024 Jul 8;15(1):5706. doi: 10.1038/s41467-024-50110-9. Nat Commun. 2024. PMID: 38977676 Free PMC article.
-
Report of the Signal Transduction Society Meeting 2018-Signaling: From Past to Future.Int J Mol Sci. 2019 Jan 8;20(1):227. doi: 10.3390/ijms20010227. Int J Mol Sci. 2019. PMID: 30626122 Free PMC article.
-
ORIGINS: A protein network-based approach to quantify cell pluripotency from scRNA-seq data.MethodsX. 2022 Jul 1;9:101778. doi: 10.1016/j.mex.2022.101778. eCollection 2022. MethodsX. 2022. PMID: 35855951 Free PMC article.
-
The CKS1/CKS2 Proteostasis Axis Is Crucial to Maintain Hematopoietic Stem Cell Function.Hemasphere. 2023 Feb 28;7(3):e853. doi: 10.1097/HS9.0000000000000853. eCollection 2023 Mar. Hemasphere. 2023. PMID: 36874381 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

