Daily Rhythms of TNFα Expression and Food Intake Regulate Synchrony of Plasmodium Stages with the Host Circadian Cycle
- PMID: 29805094
- PMCID: PMC6014587
- DOI: 10.1016/j.chom.2018.04.016
Daily Rhythms of TNFα Expression and Food Intake Regulate Synchrony of Plasmodium Stages with the Host Circadian Cycle
Abstract
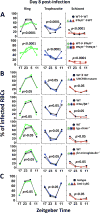
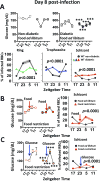
The Plasmodium cell cycle, wherein millions of parasites differentiate and proliferate, occurs in synchrony with the vertebrate host's circadian cycle. The underlying mechanisms are unknown. Here we addressed this question in a mouse model of Plasmodium chabaudi infection. Inflammatory gene expression and carbohydrate metabolism are both enhanced in interferon-γ (IFNγ)-primed leukocytes and liver cells from P. chabaudi-infected mice. Tumor necrosis factor α (TNFα) expression oscillates across the host circadian cycle, and increased TNFα correlates with hypoglycemia and a higher frequency of non-replicative ring forms of trophozoites. Conversely, parasites proliferate and acquire biomass during food intake by the host. Importantly, cyclic hypoglycemia is attenuated and synchronization of P. chabaudi stages is disrupted in IFNγ-/-, TNF receptor-/-, or diabetic mice. Hence, the daily rhythm of systemic TNFα production and host food intake set the pace for Plasmodium synchronization with the host's circadian cycle. This mechanism indicates that Plasmodium parasites take advantage of the host's feeding habits.
Keywords: IFNγ; Plasmodium; TNFα; energy metabolism; food intake; glucose; insulin; malaria and circadian cycle.
Copyright © 2018 Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors declare no competing interests.
Figures





Comment in
-
Malaria Makes the Most of Mealtimes.Cell Host Microbe. 2018 Jun 13;23(6):695-697. doi: 10.1016/j.chom.2018.05.015. Cell Host Microbe. 2018. PMID: 29902432
References
-
- Antonelli LR, Leoratti FM, Costa PA, Rocha BC, Diniz SQ, Tada MS, Pereira DB, Teixeira-Carvalho A, Golenbock DT, Goncalves R, et al. The CD14+CD16+ inflammatory monocyte subset displays increased mitochondrial activity and effector function during acute Plasmodium vivax malaria. PLoS pathogens. 2014;10:e1004393. - PMC - PubMed
-
- Bartholomeu DC, Ropert C, Melo MB, Parroche P, Junqueira CF, Teixeira SM, Sirois C, Kasperkovitz P, Knetter CF, Lien E, Latz E, et al. Recruitment and endo-lysosomal activation of TLR9 in dendritic cells Infected with Trypanosoma cruzi. Journal of immunology. 2008;181:1333–1344. - PubMed
-
- Curtis AM, Bellet MM, Sassone-Corsi P, O'Neill LA. Circadian clock proteins and immunity. Immunity. 2014;40:178–186. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

