Regulatory T Lymphocytes in Periodontitis: A Translational View
- PMID: 29805313
- PMCID: PMC5901475
- DOI: 10.1155/2018/7806912
Regulatory T Lymphocytes in Periodontitis: A Translational View
Abstract
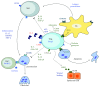
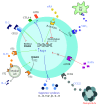
Periodontitis is a chronic immuno-inflammatory disease in which the disruption of the balance between host and microbiota interactions is key to the onset and progression of the disease. The immune homeostasis associated with periodontal health requires a regulated immuno-inflammatory response, during which the presence of regulatory T cells (Tregs) is essential to ensure a controlled response that minimizes collateral tissue damage. Since Tregs modulate both innate and adaptive immunity, pathological conditions that may resolve by the acquisition of immuno-tolerance, such as periodontitis, may benefit by the use of Treg immunotherapy. In recent years, many strategies have been proposed to take advantage of the immuno-suppressive capabilities of Tregs as immunotherapy, including the ex vivo and in vivo manipulation of the Treg compartment. Ongoing research in both basic and translational studies let us gain a better understanding of the diversity of Treg subsets, their phenotypic plasticity, and suppressive functions, which can be used as a substrate for new immunotherapies. Certainly, as our knowledge of Treg biology increases, we will be capable to develop new therapies designed to enhance the stability and function of Tregs during periodontitis.
Figures
References
-
- Sakaguchi S., Sakaguchi N., Asano M., Itoh M., Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. The Journal of Immunology. 1995;155(3):1151–1164. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

