Long-Term Progression-Free Survival in a Patient with Metastatic Leiomyosarcoma of the Inguinal Region Treated with Trabectedin
- PMID: 29805376
- PMCID: PMC5968288
- DOI: 10.1159/000487937
Long-Term Progression-Free Survival in a Patient with Metastatic Leiomyosarcoma of the Inguinal Region Treated with Trabectedin
Abstract
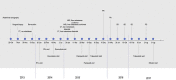
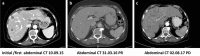
Presented here is the case of an elderly leiomyosarcoma patient with multiple comorbidities and relapses from prior lines of treatment, who experienced a long-lasting progression-free survival. After initial diagnosis, standard treatment protocols with surgery and subsequent adjuvant radiochemotherapy were administered, followed by a short course of oral pazopanib at the patient's request, which led to a rapid relapse. Afterwards, the patient received trabectedin for 22 months, achieving disease control with good quality of life over an extended period of time. After progression from trabectedin, the patient was switched to eribulin. Future clinical trials are needed to investigate the efficacy of trabectedin maintenance treatment and to identify predictive criteria for response to trabectedin among patients with advanced sarcoma.
Keywords: Alkylating agent; Leiomyosarcoma; Metastatic disease; Partial response; Progression-free survival; Soft tissue sarcoma; Trabectedin.
Figures
References
-
- Stiller CA, Trama A, Serraino D, Rossi S, Navarro C, Chirlaque MD, et al. RARECARE Working Group. Descriptive epidemiology of sarcomas in Europe: report from the RARECARE project. Eur J Cancer. 2013 Feb;49(3):684–695. - PubMed
-
- Brooks AD, Bowne WB, Delgado R, Leung DH, Woodruff J, Lewis JJ, et al. Soft tissue sarcomas of the groin: diagnosis, management, and prognosis. J Am Coll Surg. 2001 Aug;193((2)):130–136. - PubMed
-
- ESMO/European Sarcoma Network Working Group Soft tissue and visceral sarcomas: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2014 Sep;25(Suppl 3):iii102–iii112. - PubMed
-
- American Cancer Society Cancer Facts & Figures 2017 –Special Section: Rare Cancers in Adults [cited 2017 Nov 2]. Available from: https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-...
-
- Le Cesne A, Blay JY, Domont J, Tresch-Bruneel E, Chevreau C, Bertucci F, et al. Interruption versus continuation of trabectedin in patients with soft-tissue sarcoma (T-DIS): a randomised phase 2 trial. Lancet Oncol. 2015 Mar;16((3)):312–319. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

