Effect of membrane-bound complement regulatory proteins on tumor cell sensitivity to complement-dependent cytolysis triggered by heterologous expression of the α-gal xenoantigen
- PMID: 29805637
- PMCID: PMC5958734
- DOI: 10.3892/ol.2018.8478
Effect of membrane-bound complement regulatory proteins on tumor cell sensitivity to complement-dependent cytolysis triggered by heterologous expression of the α-gal xenoantigen
Abstract
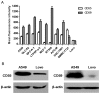
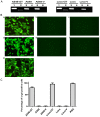
Engineering malignant cells to express a heterologous α-gal antigen can induce heterograft hyperacute rejection, resulting in complement-dependent cytolysis (CDC) of tumor cells, which has been considered as a novel strategy for antitumor therapy. A549 cells engineered to express Galα1-3Galβ1-4GlcNAc-R (α-gal) epitope exhibited strong resistance to CDC treated by normal human serum (NHS) in a previous study. We hypothesized that the expression of membrane-bound complement regulatory proteins (mCRPs) decay accelerating factor (CD55) and protectin (CD59) influenced the efficacy of the α-gal/NHS-mediated antitumor effect to tumor cells in vitro. The present study confirmed that A549 cells expressed high levels of CD55 and CD59, whereas Lovo cells expressed relatively low levels of these proteins. A549 and Lovo cells transfected with plasmids containing or lacking the α-gal epitope were evaluated for their susceptibility to CDC by NHS and detected using a trypan blue exclusion assay. α-gal-expressing Lovo (Lovo-GT) cells were almost completely killed by α-gal-mediated CDC following incubation with 50% NHS, whereas no cytolysis was observed in α-gal expressing A549 (A549-GT) cells. Abrogating CD55 and CD59 function from A549-GT cells by various concentrations of phosphatidylinositol-specific phospholipase C (PI-PLC) or blocking antibodies increased the susceptibility of cells to CDC, and the survival rate decreased significantly comparing to the controls (P<0.05). The findings of the present study indicated that using the α-gal/NHS system to eliminate tumor cells via inducing the complement cascade reaction might represent a feasible approach for the treatment of cancer. However, high levels of mCRP expression may limit the efficacy of this approach. Therefore, an improved efficacy of cancer cell killing may be achieved by combining strategies of heterologous α-gal expression and mCRP downregulation.
Keywords: complement-dependent cytolysis; membrane-bound complement regulatory proteins; α-gal epitope.
Figures


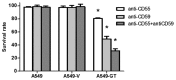
Similar articles
-
CD55 limits sensitivity to complement-dependent cytolysis triggered by heterologous expression of α-gal xenoantigen in colon tumor cells.Am J Physiol Gastrointest Liver Physiol. 2014 Jun 15;306(12):G1056-64. doi: 10.1152/ajpgi.00464.2013. Epub 2014 Apr 24. Am J Physiol Gastrointest Liver Physiol. 2014. PMID: 24763553
-
miR-132-3p regulates antibody-mediated complement-dependent cytotoxicity in colon cancer cells by directly targeting CD55.Clin Exp Immunol. 2023 Mar 8;211(1):57-67. doi: 10.1093/cei/uxac120. Clin Exp Immunol. 2023. PMID: 36571232 Free PMC article.
-
Membrane-bound complement regulatory proteins are prognostic factors of operable breast cancer treated with adjuvant trastuzumab: a retrospective study.Oncol Rep. 2014 Dec;32(6):2619-27. doi: 10.3892/or.2014.3496. Epub 2014 Sep 18. Oncol Rep. 2014. PMID: 25241923
-
Obstacles to cancer immunotherapy: expression of membrane complement regulatory proteins (mCRPs) in tumors.Mol Immunol. 2003 Sep;40(2-4):109-23. doi: 10.1016/s0161-5890(03)00112-3. Mol Immunol. 2003. PMID: 12914817 Review.
-
Xenotransplantation and ABO incompatible transplantation: the similarities they share.Transfus Apher Sci. 2006 Aug;35(1):45-58. doi: 10.1016/j.transci.2006.05.007. Epub 2006 Aug 14. Transfus Apher Sci. 2006. PMID: 16905361 Review.
Cited by
-
New Insights into Xenotransplantation for Cartilage Repair: Porcine Multi-Genetically Modified Chondrocytes as a Promising Cell Source.Cells. 2021 Aug 20;10(8):2152. doi: 10.3390/cells10082152. Cells. 2021. PMID: 34440921 Free PMC article.
-
Role of B-Cell Lymphoma 2 Ovarian Killer (BOK) in Acute Toxicity of Human Lung Epithelial Cells Caused by Cadmium Chloride.Med Sci Monit. 2019 Jul 19;25:5356-5368. doi: 10.12659/MSM.913706. Med Sci Monit. 2019. PMID: 31323016 Free PMC article.
-
AGI-134: a fully synthetic α-Gal glycolipid that converts tumors into in situ autologous vaccines, induces anti-tumor immunity and is synergistic with an anti-PD-1 antibody in mouse melanoma models.Cancer Cell Int. 2019 Dec 19;19:346. doi: 10.1186/s12935-019-1059-8. eCollection 2019. Cancer Cell Int. 2019. PMID: 31889898 Free PMC article.
-
A LY6E-PHB1-TRIM21 assembly degrades CD14 protein to mitigate LPS-induced inflammatory response.iScience. 2023 May 4;26(6):106808. doi: 10.1016/j.isci.2023.106808. eCollection 2023 Jun 16. iScience. 2023. PMID: 37250795 Free PMC article.
References
-
- Kobayashi T, Cooper DK. Anti-Gal, alpha-Gal epitopes and xenotransplantation. Subcell Biochem. 1999;32:229–257. - PubMed
-
- Eto T, Ichikawa Y, Nishimura K, Ando S, Yamakawa T. Chemistry of lipid of the posthemyolytic residue or stroma of erythrocytes. XVI. Occurrence of ceramide pentasaccharide in the membrane of erythrocytes and reticulocytes of rabbit. J Biochem. 1968;64:205–213. doi: 10.1093/oxfordjournals.jbchem.a128881. - DOI - PubMed
-
- Galili U, Shohet SB, Kobrin E, Stults CL, Macher BA. Man, apes, and Old World monkeys differ from other mammals in the expression of alpha-galactosyl epitopes on nucleated cells. J Biol Chem. 1988;263:17755–17762. - PubMed
-
- Roy BB, Jinno-Oue A, Shinagawa M, Shimizu A, Tamura K, Shimizu N, Tanaka A, Hoshino H. Isolation of the feline alpha1,3-galactosyltransferase gene, expression in transfected human cells and its phylogenetic analysis. J Exp Zool B Mol Dev Evol. 2006;306:59–69. doi: 10.1002/jez.b.21072. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
