Exosome-encapsulated microRNA-223-3p as a minimally invasive biomarker for the early detection of invasive breast cancer
- PMID: 29805680
- PMCID: PMC5958689
- DOI: 10.3892/ol.2018.8457
Exosome-encapsulated microRNA-223-3p as a minimally invasive biomarker for the early detection of invasive breast cancer
Abstract
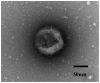
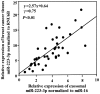
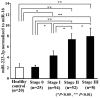
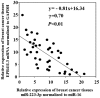
Patients diagnosed preoperatively with ductal carcinoma in situ (DCIS) breast cancer have the potential to develop invasive ductal carcinoma (IDC). The present study investigated the usefulness of exosome-encapsulated microRNA-223-3p (miR-223-3p) as a biomarker for detecting IDC in patients initially diagnosed with DCIS by biopsy. The potential association between miR-223-3p and clinicopathological characteristics was examined in patients with breast cancer. Exosomes of 185 patients with breast cancer were separated from plasma by ultracentrifugation. Initially a microRNA (miRNA) microarray was examined to reveal the invasion specific miRNAs using exosomes collected from 6 patients with breast cancer, including 3 DCIS patients, 3 IDC patients and 3 healthy controls. In the miR microarray analysis the miR-223-3p levels of IDC patients demonstrated the highest fold-change compared with those in the DCIS patients and healthy controls. The potential of miR-223-3p for cell proliferation and cell invasion were examined in vitro using MCF7 cells transfected with the miR-223-3p gene. MCF7 cells transfected with the miR-223-3p gene significantly promoted cell proliferation and cell invasive ability (P<0.05). The plasma exosomal miR-223-3p levels of the other 179 patients with breast cancer and 20 healthy controls were measured using TaqMan miR assays. The exosomal miR-223-3p levels of the patients with breast cancer were significantly increased compared with the healthy controls (P<0.01). A statistically significant association was observed between the exosomal miR-223-3p levels and histological type, pT stage, pN stage, pathological stage, lymphatic invasion and nuclear grade (P<0.05). The exosomal miR-223-3p levels of IDC patients (stage I) and upstaged IDC patients (stage I) were significantly higher compared with the DCIS patients (P<0.05). These results suggest that exosomal miR-223-3p may be a useful preoperative biomarker to identify the invasive lesions of DCIS patients diagnosed by biopsy. In addition, plasma exosome-encapsulated miR-223-3p level was significantly associated with the malignancy of breast cancer.
Keywords: biomarker; breast cancer; detecting invasive ductal carcinoma; ductal carcinoma in situ; exosome; microRNA-223-3p.
Figures



References
-
- Foundation for Promotion of Cancer Research. National Cancer Center; Tokyo: 2016. [Mar 30;2016 ]. The Editorial Board of the Cancer Statistics in Japan: Cancer Statistics in Japan 2015.
-
- Fentiman IS. The dilemma of in situ carcinoma of the breast. Int J Clin Pract. 2001;55:680–683. - PubMed
-
- Cox CE, Nguyen K, Gray RJ, Salud C, Ku NN, Dupont E, Hutson L, Peltz E, Whitehead G, Reintgen D, Cantor A. Importance of lymphatic mapping in ductal carcinoma in situ (DCIS): Why map DCIS? Am Surg. 2001;67:513–519. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
