Multivalent Antigen Presentation Enhances the Immunogenicity of a Synthetic Three-Component HIV-1 V3 Glycopeptide Vaccine
- PMID: 29806004
- PMCID: PMC5968512
- DOI: 10.1021/acscentsci.8b00060
Multivalent Antigen Presentation Enhances the Immunogenicity of a Synthetic Three-Component HIV-1 V3 Glycopeptide Vaccine
Abstract
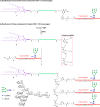
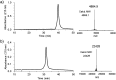
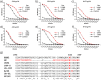
HIV-1 envelope glycoproteins gp120 and gp41 are presented on the virus surface as a trimer of heterodimer and are the targets of broadly neutralizing antibodies (bNAbs). We describe here the synthesis and preliminary immunological evaluation of a three-component trivalent HIV-1 V3 glycopeptide immunogen aiming to raise glycopeptide epitope-specific antibodies. Click chemistry confers efficient synthesis of the lipopeptide-glycopeptide conjugate that carries three copies of HIV-1 JR-FL gp120 V3 glycopeptide with a high-mannose glycan at the N332 glycosylation site. We found that the multivalent presentation substantially enhanced the immunogenicity of the V3 glycopeptide. The antisera induced by the three-component multivalent glycopeptide immunogen exhibited stronger binding to heterologous HIV-1 gp120s and the trimeric gp140s than that from the monovalent glycopeptide immunogen. The antisera generated from this preliminary rabbit immunization did not show virus neutralization activity, probably due to the lack of somatic maturation. The ability to elicit substantial glycopeptide epitope-specific antibodies by the three-component trivalent glycopeptide immunogen suggests that it could serve as a valuable vaccine component in combination with other vaccine candidates for further immunization studies.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

References
-
- Stewart-Jones G. B.; Soto C.; Lemmin T.; Chuang G. Y.; Druz A.; Kong R.; Thomas P. V.; Wagh K.; Zhou T.; Behrens A. J.; Bylund T.; Choi C. W.; Davison J. R.; Georgiev I. S.; Joyce M. G.; Kwon Y. D.; Pancera M.; Taft J.; Yang Y.; Zhang B.; Shivatare S. S.; Shivatare V. S.; Lee C. C.; Wu C. Y.; Bewley C. A.; Burton D. R.; Koff W. C.; Connors M.; Crispin M.; Baxa U.; Korber B. T.; Wong C. H.; Mascola J. R.; Kwong P. D. Trimeric HIV-1-Env Structures Define Glycan Shields from Clades A, B, and G. Cell 2016, 165, 813–826. 10.1016/j.cell.2016.04.010. - DOI - PMC - PubMed
-
- Pancera M.; Zhou T.; Druz A.; Georgiev I. S.; Soto C.; Gorman J.; Huang J.; Acharya P.; Chuang G. Y.; Ofek G.; Stewart-Jones G. B.; Stuckey J.; Bailer R. T.; Joyce M. G.; Louder M. K.; Tumba N.; Yang Y.; Zhang B.; Cohen M. S.; Haynes B. F.; Mascola J. R.; Morris L.; Munro J. B.; Blanchard S. C.; Mothes W.; Connors M.; Kwong P. D. Structure and immune recognition of trimeric pre-fusion HIV-1 Env. Nature 2014, 514, 455–461. 10.1038/nature13808. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

