[Effect of short-term low-frequency electrical stimulation on nerve regeneration of delayed nerve defect during operation]
- PMID: 29806265
- PMCID: PMC8458119
- DOI: 10.7507/1002-1892.201609024
[Effect of short-term low-frequency electrical stimulation on nerve regeneration of delayed nerve defect during operation]
Abstract
Objective: To explore the effect of short-term low-frequency electrical stimulation (SLES) during operation on nerve regeneration in delayed peripheral nerve injury with long gap.
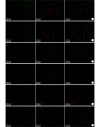
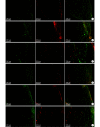
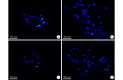
Methods: Thirty female adult Sprague Dawley rats, weighing 160-180 g, were used to prepare 13-mm defect model by trimming the nerve stumps. Then all rats were randomly divided into 2 groups, 15 rats in each group. After nerve defect was bridged by the contralateral normal sciatic nerve, SLES was applied in the experimental group, but was not in the control group. The spinal cords and dorsal root ganglions (DRGs) were harvested to carry out immunofluorescence histochemistry double staining for growth-associated proteins 43 (GAP-43) and brain-derived neurotrophic factor (BDNF) at 1, 2, and 7 days after repair. Fluorogold (FG) retrograde tracing was performed at 3 months after repair. The mid-portion regenerated segments were harvested to perform Meyer's trichrome staining, immunofluorescence double staining for neurofilament (NF) and soluble protein 100 (S-100) on the transversely or longitudinal sections at 3 months after repair. The segment of the distal sciatic nerve trunk was harvested for electron microscopy and morphometric analyses to measure the diameter of the myelinated axons, thickness of myelin sheaths, the G ratio, and the density of the myelinated nerve fibers. The gastrocnemius muscles of the operated sides were harvested to measure the relative wet weight ratios. Karnovsky-Root cholinesterase staining of the motor endplate was carried out.
Results: In the experimental group, the expressions of GAP-43 and BDNF were higher than those in the control group at 1 and 2 days after repair. The number of labeled neurons in the anterior horn of gray matter in the spinal cord and DRGs at the operated side from the experimental group was more than that from the control group. Meyer's trichrome staining, immunofluorescence double staining, and the electron microscopy observation showed that the regenerated nerves were observed to develop better in the experimental group than the control group. The relative wet weight ratio of experimental group was significantly higher than that of the control group ( t=4.633, P=0.000). The size and the shape of the motor endplates in the experimental group were better than those in the control group.
Conclusion: SLES can promote the regeneration ability of the short-term (1 month) delayed nerve injury with long gap to a certain extent.
目的: 探讨术中短期低频电刺激(short-term low-frequency electrical stimulation,SLES)对陈旧性周围神经缺损再生能力的影响。.
方法: 成年雌性 SD 大鼠 30 只,体质量 160~180 g,制备大鼠坐骨神经 13 mm 长陈旧性缺损模型,随机分为实验组和对照组,每组 15 只。实验组切取对侧正常坐骨神经桥接缺损并施以 SLES,对照组同法修复神经缺损但不予以电刺激。修复术后 1、2、7 d 取大鼠脊髓腰膨大段(L 4、5)以及相应背根神经节,行抗生长相关蛋白 43(growth-associated proteins 43,GAP-43)及抗脑源性神经营养因子(brain-derived neurotrophic factor,BDNF)双重免疫荧光染色观察。修复术后 3 个月行荧光金逆行示踪实验。取再生神经组织中段作横、纵冰冻切片,分别行 Meyer 神经三色染色和抗鼠神经丝蛋白(neurofilament,NF)及可溶性蛋白 100(soluble protein 100,S-100)双重免疫荧光染色观察,并取术侧远端神经行透射电镜观察,测量有髓神经纤维、轴突直径及髓鞘厚度,计算有髓神经纤维密度及 G 比值。取术侧腓肠肌计算相对湿重比,并行 Karnovsky-Root 运动终板胆碱酯酶组织学染色观察。.
结果: 实验组修复术后 1、2 d,术侧对应运动神经元和感觉神经元 GAP-43 和 BDNF 表达上调快于对照组。荧光金逆行示踪实验示,实验组术侧脊髓前角及相应背根神经节中标记的神经元多于对照组。实验组再生神经 Meyer 神经三色染色和双重免疫荧光染色均显示再生神经组织发育优于对照组。透射电镜观察示再生神经纤维成簇分布,实验组轴突直径、髓鞘厚度、有髓神经纤维密度及 G 比值均高于对照组,但差异无统计学意义( P>0.05)。实验组术侧腓肠肌相对湿重比高于对照组,差异有统计学意义( t=4.633, P=0.000)。腓肠肌 Karnovsky-Root 运动终板胆碱酯酶组织学染色示,实验组运动终板的形态和数量略优于对照组。.
结论: 术中 SLES 能在一定程度上提高大鼠短期(1 个月)长距离陈旧性周围神经缺损的再生能力。.
Keywords: Short-time low-frequency electrical stimulation; chronic injury; nerve defect repair; peripheral nerve; rat.
Figures





Similar articles
-
[Promotion of transplanted collagen scaffolds combined with brain-derived neurotrophic factor for axonal regeneration and motor function recovery in rats after transected spinal cord injury].Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2018 Jun 15;32(6):650-659. doi: 10.7507/1002-1892.201803094. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2018. PMID: 29905040 Free PMC article. Chinese.
-
Electrical stimulation promotes regeneration of defective peripheral nerves after delayed repair intervals lasting under one month.PLoS One. 2014 Sep 2;9(9):e105045. doi: 10.1371/journal.pone.0105045. eCollection 2014. PLoS One. 2014. PMID: 25181499 Free PMC article.
-
Electrical stimulation accelerates nerve regeneration and functional recovery in delayed peripheral nerve injury in rats.Eur J Neurosci. 2013 Dec;38(12):3691-701. doi: 10.1111/ejn.12370. Epub 2013 Oct 10. Eur J Neurosci. 2013. PMID: 24118464
-
[Morphology research of the rat sciatic nerve bridged by collage-heparin sulfate scaffold].Zhonghua Wai Ke Za Zhi. 2005 Apr 15;43(8):531-4. Zhonghua Wai Ke Za Zhi. 2005. PMID: 15938914 Chinese.
-
The role of neurotrophic factors in nerve regeneration.Neurosurg Focus. 2009 Feb;26(2):E3. doi: 10.3171/FOC.2009.26.2.E3. Neurosurg Focus. 2009. PMID: 19228105 Review.
References
-
- Jiao HS, Yao J, Yang Y Chitosan/polyglycolic acid nerve grafts for axon regeneration from prolonged axotomized neurons to chronically denervated segments. Biomaterials. 2009;30(28):5004–5018. - PubMed
-
- Furey MJ, Midha R, Xu QG Prolonged target deprivation reduces the capacity of injured motoneurons to regenerate. Neurosurgery. 2007;60(4):723–733. - PubMed
-
- Gordon T, Brushart TM, Amirjani N The potential of electrical stimulation to promote functional recovery after peripheral nerve injury—comparisons between rats and humans. Acta Neurochir Suppl. 2007;100:3–11. - PubMed
-
- Gordon T, Amirjani N, Edwards DC , Brief post-surgical electrical stimulation accelerates axon regeneration and muscle reinnervation without affecting the functional measures in carpal tunnel syndrome patients. Exp Neurol 2010;223(1):192–202. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous
