Light-Gated Rotation in a Molecular Motor Functionalized with a Dithienylethene Switch
- PMID: 29806875
- PMCID: PMC6099277
- DOI: 10.1002/anie.201802392
Light-Gated Rotation in a Molecular Motor Functionalized with a Dithienylethene Switch
Abstract
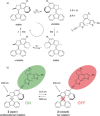
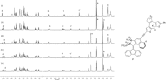
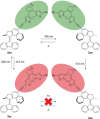
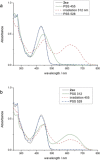
A multiphotochromic hybrid system is presented in which a light-driven overcrowded alkene-based molecular rotary motor is connected to a dithienylethene photoswitch. Ring closing of the dithienylethene moiety, using an irradiation wavelength different from the wavelength applied to operate the molecular motor, results in inhibition of the rotary motion as is demonstrated by detailed 1 H-NMR and UV/Vis experiments. For the first time, a light-gated molecular motor is thus obtained. Furthermore, the excitation wavelength of the molecular motor is red-shifted from the UV into the visible-light region upon attachment of the dithienylethene switch.
Keywords: alkenes; diarylethenes; molecular motors; molecular switches; photochromism.
© 2018 The Authors. Published by Wiley-VCH Verlag GmbH & Co. KGaA.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Coupled Rotary and Oscillatory Motion in a Second-Generation Molecular Motor Pd Complex.J Am Chem Soc. 2023 Jan 18;145(2):822-829. doi: 10.1021/jacs.2c08267. Epub 2023 Jan 5. J Am Chem Soc. 2023. PMID: 36603116 Free PMC article.
-
Visible-Light-Driven Photoisomerization and Increased Rotation Speed of a Molecular Motor Acting as a Ligand in a Ruthenium(II) Complex.Angew Chem Int Ed Engl. 2015 Sep 21;54(39):11457-61. doi: 10.1002/anie.201505781. Epub 2015 Aug 13. Angew Chem Int Ed Engl. 2015. PMID: 26271465
-
Braking of a Light-Driven Molecular Rotary Motor by Chemical Stimuli.Chemistry. 2018 Jan 2;24(1):81-84. doi: 10.1002/chem.201704747. Epub 2017 Nov 20. Chemistry. 2018. PMID: 29154435
-
Visible Light-Driven Molecular Switches and Motors: Recent Developments and Applications.Chemistry. 2022 Mar 28;28(18):e202103906. doi: 10.1002/chem.202103906. Epub 2022 Jan 28. Chemistry. 2022. PMID: 34964995 Review.
-
Chemically Driven Rotatory Molecular Machines.Angew Chem Int Ed Engl. 2022 Oct 4;61(40):e202206631. doi: 10.1002/anie.202206631. Epub 2022 Sep 5. Angew Chem Int Ed Engl. 2022. PMID: 35852813 Free PMC article. Review.
Cited by
-
Designing light-driven rotary molecular motors.Chem Sci. 2021 Oct 20;12(45):14964-14986. doi: 10.1039/d1sc04781g. eCollection 2021 Nov 24. Chem Sci. 2021. PMID: 34909140 Free PMC article. Review.
-
Responsive Molecules for Organic Neuromorphic Devices: Harnessing Memory Diversification.Adv Mater. 2025 May;37(19):e2418281. doi: 10.1002/adma.202418281. Epub 2025 Mar 26. Adv Mater. 2025. PMID: 40135253 Free PMC article. Review.
-
Dual-function artificial molecular motors performing rotation and photoluminescence.Sci Adv. 2022 Nov 4;8(44):eadd0410. doi: 10.1126/sciadv.add0410. Epub 2022 Nov 4. Sci Adv. 2022. PMID: 36332022 Free PMC article.
-
Theoretical study on pentiptycene molecular brake: photoinduced isomerization and photoinduced electron transfer.J Mol Model. 2021 Sep 18;27(10):289. doi: 10.1007/s00894-021-04900-3. J Mol Model. 2021. PMID: 34536143
-
Designing a visible light-mediated double photoswitch: a combination of biradical and azobenzene structural motifs that can be switched independently.Chem Sci. 2024 Dec 3;16(2):876-888. doi: 10.1039/d4sc07247b. eCollection 2025 Jan 2. Chem Sci. 2024. PMID: 39660294 Free PMC article.
References
-
- Sauvage J.-P., Acc. Chem. Res. 1998, 31, 611–619.
-
- Balzani V., Credi A., Raymo F. M., Stoddart J. F., Angew. Chem. Int. Ed. 2000, 39, 3348–3391; - PubMed
- Angew. Chem. 2000, 112, 3484–3530.
-
- Browne W. R., Feringa B. L., Nat. Nanotechnol. 2006, 1, 25–35. - PubMed
-
- Molecular Devices and Machines: Concepts and Perspectives for the Nanoworld, Second ed (Eds.: V. Balzani, A. Credi, M. Venturi), Wiley-VCH, Weinheim, 2008.
-
- Coskun A., Banaszak M., Astumian R. D., Stoddart J. F., Grzybowski B. A., Chem. Soc. Rev. 2012, 41, 19–30. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

