Health and economic benefits of single-dose HPV vaccination in a Gavi-eligible country
- PMID: 29807710
- PMCID: PMC6066173
- DOI: 10.1016/j.vaccine.2018.04.061
Health and economic benefits of single-dose HPV vaccination in a Gavi-eligible country
Abstract
Background: Although guidelines for prophylactic human papillomavirus (HPV) vaccination recommend two doses for girls ages 9-14 years, several studies have demonstrated similar protection with one dose. Our objective was to evaluate the long-term health and economic impacts of routine one-dose HPV vaccination compared to (1) no vaccination and (2) two-dose HPV vaccination in a low-income country.
Methods: We used a three-tiered hybrid modeling approach that captured HPV transmission, cervical carcinogenesis, and population demographics to project long-term health and economic outcomes associated with one-dose HPV vaccination (assuming 80% efficacy against HPV-16/18 infections under three waning scenarios) and two-dose HPV vaccination (assuming 100% efficacy over the lifetime) in Uganda. Costs included the vaccine program (dosage and delivery) costs over a 10-year period and cervical cancer costs over the lifetimes of the current population of Ugandan women. Health outcomes included number of cervical cancer cases and disability-adjusted life years (DALYs). Incremental cost-effectiveness ratios (i.e., cost per DALY averted) were calculated and compared against the Ugandan per-capita gross domestic product.
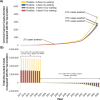
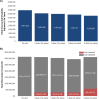
Results: Routine one-dose HPV vaccination of 9-year-old girls required substantial upfront investment but was cost-saving compared to no vaccination when accounting for the cost-offsets from future cancers averted. Forty years after initiating routine vaccination and depending on assumptions of vaccine waning, one-dose HPV vaccination with equivalent coverage (70%) averted 15-16% of cervical cancer cases versus 21% with two-dose vaccination but required only half the upfront economic investment. Vaccination with two doses had an attractive cost-effectiveness profile except if one-dose vaccination enabled higher coverage (90% vs. 70%) and did not wane.
Conclusions: One-dose HPV vaccination resulted in cost-savings compared to no vaccination and could be cost-effective compared to two-dose vaccination if protection is longstanding and higher coverage can be achieved.
Keywords: Cervical cancer; Human papillomavirus; Vaccination.
Copyright © 2018. Published by Elsevier Ltd.
Figures

References
-
- Schiffman M., Doorbar J., Wentzensen N., de Sanjose S., Fakhry C., Monk B.J. Carcinogenic human papillomavirus infection. Nat Rev Dis Primers. 2016;2:16086. - PubMed
-
- World Health Organization & International Agency for Research on Cancer. IARC Monographs on the evaluation of carcinogenic risks to humans; 2007.
-
- World Health Organization. Weekly epidemiological record; 2017. p. 241–68.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

