Mesenchymal Stem Cell-Based Therapy for Cardiovascular Disease: Progress and Challenges
- PMID: 29807782
- PMCID: PMC6037203
- DOI: 10.1016/j.ymthe.2018.05.009
Mesenchymal Stem Cell-Based Therapy for Cardiovascular Disease: Progress and Challenges
Abstract
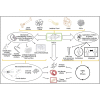
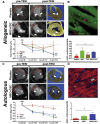
Administration of mesenchymal stem cells (MSCs) to diseased hearts improves cardiac function and reduces scar size. These effects occur via the stimulation of endogenous repair mechanisms, including regulation of immune responses, tissue perfusion, inhibition of fibrosis, and proliferation of resident cardiac cells, although rare events of transdifferentiation into cardiomyocytes and vascular components are also described in animal models. While these improvements demonstrate the potential of stem cell therapy, the goal of full cardiac recovery has yet to be realized in either preclinical or clinical studies. To reach this goal, novel cell-based therapeutic approaches are needed. Ongoing studies include cell combinations, incorporation of MSCs into biomaterials, or pre-conditioning or genetic manipulation of MSCs to boost their release of paracrine factors, such as exosomes, growth factors, microRNAs, etc. All of these approaches can augment therapeutic efficacy. Further study of the optimal route of administration, the correct dose, the best cell population(s), and timing for treatment are parameters that still need to be addressed in order to achieve the goal of complete cardiac regeneration. Despite significant progress, many challenges remain.
Keywords: cardiovascular disease; mesenchymal stem cell; regenerative medicine.
Copyright © 2018. Published by Elsevier Inc.
Figures

Comment in
-
Medicinal signalling cells: they work, so use them.Nature. 2019 Feb;566(7742):39. doi: 10.1038/d41586-019-00490-6. Nature. 2019. PMID: 30723355 No abstract available.
References
-
- Becker C., Lacchini S., Muotri A.R., da Silva G.J., Castelli J.B., Vassallo P.F., Menck C.F., Krieger J.E. Skeletal muscle cells expressing VEGF induce capillary formation and reduce cardiac injury in rats. Int. J. Cardiol. 2006;113:348–354. - PubMed
-
- Post M.J., Sato K., Murakami M., Bao J., Tirziu D., Pearlman J.D., Simons M. Adenoviral PR39 improves blood flow and myocardial function in a pig model of chronic myocardial ischemia by enhancing collateral formation. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006;290:R494–R500. - PubMed
-
- Hedman M., Hartikainen J., Syvänne M., Stjernvall J., Hedman A., Kivelä A., Vanninen E., Mussalo H., Kauppila E., Simula S. Safety and feasibility of catheter-based local intracoronary vascular endothelial growth factor gene transfer in the prevention of postangioplasty and in-stent restenosis and in the treatment of chronic myocardial ischemia: phase II results of the Kuopio Angiogenesis Trial (KAT) Circulation. 2003;107:2677–2683. - PubMed
-
- Henry T.D., Annex B.H., McKendall G.R., Azrin M.A., Lopez J.J., Giordano F.J., Shah P.K., Willerson J.T., Benza R.L., Berman D.S., VIVA Investigators The VIVA trial: Vascular endothelial growth factor in Ischemia for Vascular Angiogenesis. Circulation. 2003;107:1359–1365. - PubMed
-
- Byrne M.J., Power J.M., Preovolos A., Mariani J.A., Hajjar R.J., Kaye D.M. Recirculating cardiac delivery of AAV2/1SERCA2a improves myocardial function in an experimental model of heart failure in large animals. Gene Ther. 2008;15:1550–1557. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

