Proteins and antibodies in serum, plasma, and whole blood-size characterization using asymmetrical flow field-flow fractionation (AF4)
- PMID: 29808297
- PMCID: PMC6061777
- DOI: 10.1007/s00216-018-1127-2
Proteins and antibodies in serum, plasma, and whole blood-size characterization using asymmetrical flow field-flow fractionation (AF4)
Abstract
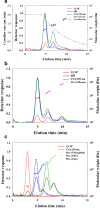
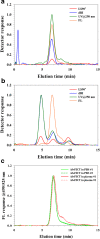
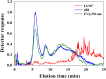
The analysis of aggregates of therapeutic proteins is crucial in order to ensure efficacy and patient safety. Typically, the analysis is performed in the finished formulation to ensure that aggregates are not present. An important question is, however, what happens to therapeutic proteins, with regard to oligomerization and aggregation, after they have been administrated (i.e., in the blood). In this paper, the separation of whole blood, plasma, and serum is shown using asymmetric flow field-flow fractionation (AF4) with a minimum of sample pre-treatment. Furthermore, the analysis and size characterization of a fluorescent antibody in blood plasma using AF4 are demonstrated. The results show the suitability and strength of AF4 for blood analysis and open new important routes for the analysis and characterization of therapeutic proteins in the blood.
Keywords: Antibodies; Asymmetric flow field-flow fractionation (AF4); Fluorescence labelling; Plasma; Serum; Whole blood.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
References
-
- Manning RR, Holcomb RE, Wilson GA, Henry CS, Manning MC. Review of orthogonal methods to SEC for quantitation and characterization of protein aggregates. Biopharm Int. 2014;27(12):32.
-
- Carpenter JF, Randolph TW, Jiskoot W, Crommelin DJA, Middaugh CR, Winter G. Potential inaccurate quantitation and sizing of protein aggregates by size exclusion chromatography: essential need to use orthogonal methods to assure the quality of therapeutic protein products. J Pharm Sci. 2010;99(5):2200–2208. doi: 10.1002/jps.21989. - DOI - PubMed
-
- USFDA (2014) Guidance for industry immunogenicity assessment for therapeutic protein products.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

