Loss of androgen receptor signaling in prostate cancer-associated fibroblasts (CAFs) promotes CCL2- and CXCL8-mediated cancer cell migration
- PMID: 29808619
- PMCID: PMC6068356
- DOI: 10.1002/1878-0261.12327
Loss of androgen receptor signaling in prostate cancer-associated fibroblasts (CAFs) promotes CCL2- and CXCL8-mediated cancer cell migration
Abstract
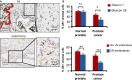
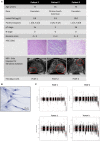
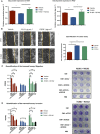
Fibroblasts are abundantly present in the prostate tumor microenvironment (TME), including cancer-associated fibroblasts (CAFs) which play a key role in cancer development. Androgen receptor (AR) signaling is the main driver of prostate cancer (PCa) progression, and stromal cells in the TME also express AR. High-grade tumor and poor clinical outcome are associated with low AR expression in the TME, which suggests a protective role of AR signaling in the stroma against PCa development. However, the mechanism of this relation is not clear. In this study, we isolated AR-expressing CAF-like cells. Testosterone (R1881) exposure did not affect CAF-like cell morphology, proliferation, or motility. PCa cell growth was not affected by culturing in medium from R1881-exposed CAF-like cells; however, migration of PCa cells was inhibited. AR chromatin immune precipitation sequencing (ChIP-seq) was performed and motif search suggested that AR in CAF-like cells bound the chromatin through AP-1-elements upon R1881 exposure, inducing enhancer-mediated AR chromatin interactions. The vast majority of chromatin binding sites in CAF-like cells were unique and not shared with AR sites observed in PCa cell lines or tumors. AR signaling in CAF-like cells decreased expression of multiple cytokines; most notably CCL2 and CXCL8 and both cytokines increased migration of PCa cells. These results suggest direct paracrine regulation of PCa cell migration by CAFs through AR signaling.
Keywords: CCL2; CXCL8; EMT; androgen receptor; cancer cell migration; cancer-associated fibroblasts; prostate cancer.
© 2018 The Authors. Published by FEBS Press and John Wiley & Sons Ltd.
Figures



Similar articles
-
Androgen receptor in cancer-associated fibroblasts influences stemness in cancer cells.Endocr Relat Cancer. 2017 Apr;24(4):157-170. doi: 10.1530/ERC-16-0138. Endocr Relat Cancer. 2017. PMID: 28264911 Free PMC article.
-
Interleukin-6 induces VEGF secretion from prostate cancer cells in a manner independent of androgen receptor activation.Prostate. 2018 Aug;78(11):849-856. doi: 10.1002/pros.23643. Epub 2018 Apr 29. Prostate. 2018. PMID: 29707793
-
Androgen receptor moonlighting in the prostate cancer microenvironment.Endocr Relat Cancer. 2018 Jun;25(6):R331-R349. doi: 10.1530/ERC-18-0042. Epub 2018 Apr 4. Endocr Relat Cancer. 2018. PMID: 29618577
-
LMO2 upregulation due to AR deactivation in cancer-associated fibroblasts induces non-cell-autonomous growth of prostate cancer after androgen deprivation.Cancer Lett. 2021 Apr 10;503:138-150. doi: 10.1016/j.canlet.2021.01.017. Epub 2021 Jan 24. Cancer Lett. 2021. PMID: 33503448
-
Androgen action in the prostate gland.Minerva Urol Nefrol. 2012 Mar;64(1):35-49. Minerva Urol Nefrol. 2012. PMID: 22402316 Review.
Cited by
-
Cancer-Associated Fibroblast: Role in Prostate Cancer Progression to Metastatic Disease and Therapeutic Resistance.Cells. 2023 Mar 4;12(5):802. doi: 10.3390/cells12050802. Cells. 2023. PMID: 36899938 Free PMC article. Review.
-
Molecular mechanisms and targeted therapy for the metastasis of prostate cancer to the bones (Review).Int J Oncol. 2024 Nov;65(5):104. doi: 10.3892/ijo.2024.5692. Epub 2024 Sep 20. Int J Oncol. 2024. PMID: 39301646 Free PMC article. Review.
-
Agent-based modeling of the prostate tumor microenvironment uncovers spatial tumor growth constraints and immunomodulatory properties.NPJ Syst Biol Appl. 2024 Feb 21;10(1):20. doi: 10.1038/s41540-024-00344-6. NPJ Syst Biol Appl. 2024. PMID: 38383542 Free PMC article.
-
"Stromal cells in prostate cancer pathobiology: friends or foes?".Br J Cancer. 2023 Apr;128(6):930-939. doi: 10.1038/s41416-022-02085-x. Epub 2022 Dec 8. Br J Cancer. 2023. PMID: 36482187 Free PMC article. Review.
-
Transcription factors in fibroblast plasticity and CAF heterogeneity.J Exp Clin Cancer Res. 2023 Dec 20;42(1):347. doi: 10.1186/s13046-023-02934-4. J Exp Clin Cancer Res. 2023. PMID: 38124183 Free PMC article. Review.
References
-
- Araki S, Omori Y, Lyn D, Singh RK, Meinbach DM, Sandman Y, Lokeshwar VB and Lokeshwar BL (2007) Interleukin‐8 is a molecular determinant of androgen independence and progression in prostate cancer. Cancer Res 67, 6854–6862. - PubMed
-
- Armstrong CW, Maxwell PJ, Ong CW, Redmond KM, McCann C, Neisen J, Ward GA, Chessari G, Johnson C, Crawford NT et al (2016) PTEN deficiency promotes macrophage infiltration and hypersensitivity of prostate cancer to IAP antagonist/radiation combination therapy. Oncotarget 7, 7885–7898. - PMC - PubMed
-
- Chawla S, Wang S, Kim S, Sheriff S, Lee P, Rengan R, Lin A, Melhem E, Maudsley A and Poptani H (2013) Radiation injury to the normal brain measured by 3D‐echo‐planar spectroscopic imaging and diffusion tensor imaging: initial experience. J Neuroimaging, 25, 97–104. - PubMed
-
- Cunha GR, Hayward SW, Dahiya R and Foster BA (1996) Smooth muscle‐epithelial interactions in normal and neoplastic prostatic development. Acta Anat (Basel) 155, 63–72. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

