111In-labelled polymeric nanoparticles incorporating a ruthenium-based radiosensitizer for EGFR-targeted combination therapy in oesophageal cancer cells
- PMID: 29808844
- PMCID: PMC5994990
- DOI: 10.1039/c7nr09606b
111In-labelled polymeric nanoparticles incorporating a ruthenium-based radiosensitizer for EGFR-targeted combination therapy in oesophageal cancer cells
Abstract
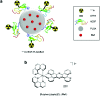
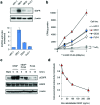
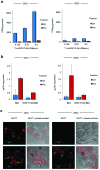
Radiolabelled, drug-loaded nanoparticles may combine the theranostic properties of radionuclides, the controlled release of chemotherapy and cancer cell targeting. Here, we report the preparation of poly(lactic-co-glycolic acid) (PLGA) nanoparticles surface conjugated to DTPA-hEGF (DTPA = diethylenetriaminepentaacetic acid, hEGF = human epidermal growth factor) and encapsulating the ruthenium-based DNA replication inhibitor and radiosensitizer Ru(phen)2(tpphz)2+ (phen = 1,10-phenanthroline, tpphz = tetrapyridophenazine) Ru1. The functionalized PLGA surface incorporates the metal ion chelator DTPA for radiolabelling and the targeting ligand for EGF receptor (EGFR). Nanoparticles radiolabelled with 111In are taken up preferentially by EGFR-overexpressing oesophageal cancer cells, where they exhibit radiotoxicity through the generation of cellular DNA damage. Moreover, nanoparticle co-delivery of Ru1 alongside 111In results in decreased cell survival compared to single-agent formulations; an effect that occurs through DNA damage enhancement and an additive relationship between 111In and Ru1. Substantially decreased uptake and radiotoxicity of nanoparticles towards normal human fibroblasts and oesophageal cancer cells with normal EGFR levels is observed. This work demonstrates nanoparticle co-delivery of a therapeutic radionuclide plus a ruthenium-based radiosensitizer can achieve combinational and targeted therapeutic effects in cancer cells that overexpress EGFR.
Figures




References
-
- Wei Q., Chen L., Sheng L., Nordgren H., Wester K., Carlsson J. Int. J. Oncol. 2007;31:493. - PubMed
-
- Gibson M. K., Abraham S. C., Wu T. T., Burtness B., Heitmiller R. F., Heath E., Forastiere A. Clin. Cancer Res. 2003;9:6461. - PubMed
-
- Liang K., Ang K. K., Milas L., Hunter N., Fan Z. Int. J. Radiat. Oncol., Biol., Phys. 2003;57:246. - PubMed
-
- Wang V. E., Grandis J. R., Ko A. H. Clin. Cancer Res. 2016;22:4283. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

