Functional and structural characterization of an ECF-type ABC transporter for vitamin B12
- PMID: 29809140
- PMCID: PMC5997447
- DOI: 10.7554/eLife.35828
Functional and structural characterization of an ECF-type ABC transporter for vitamin B12
Abstract
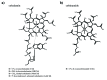
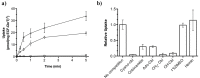
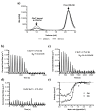
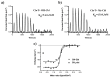
Vitamin B12 (cobalamin) is the most complex B-type vitamin and is synthetized exclusively in a limited number of prokaryotes. Its biologically active variants contain rare organometallic bonds, which are used by enzymes in a variety of central metabolic pathways such as L-methionine synthesis and ribonucleotide reduction. Although its biosynthesis and role as co-factor are well understood, knowledge about uptake of cobalamin by prokaryotic auxotrophs is scarce. Here, we characterize a cobalamin-specific ECF-type ABC transporter from Lactobacillus delbrueckii, ECF-CbrT, and demonstrate that it mediates the specific, ATP-dependent uptake of cobalamin. We solved the crystal structure of ECF-CbrT in an apo conformation to 3.4 Å resolution. Comparison with the ECF transporter for folate (ECF-FolT2) from the same organism, reveals how the identical ECF module adjusts to interact with the different substrate binding proteins FolT2 and CbrT. ECF-CbrT is unrelated to the well-characterized B12 transporter BtuCDF, but their biochemical features indicate functional convergence.
Keywords: E. coli; biochemistry; chemical biology; membrane transport; molecular biophysics; structural biology; transport mechanism; vitamin B12; vitamin trasnport.
© 2018, Santos et al.
Conflict of interest statement
JS, SR, SM, CP, Jt, Jd, AG, DS No competing interests declared
Figures




References
-
- Adams PD, Afonine PV, Bunkóczi G, Chen VB, Davis IW, Echols N, Headd JJ, Hung LW, Kapral GJ, Grosse-Kunstleve RW, McCoy AJ, Moriarty NW, Oeffner R, Read RJ, Richardson DC, Richardson JS, Terwilliger TC, Zwart PH. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallographica Section D Biological Crystallography. 2010;66:213–221. doi: 10.1107/S0907444909052925. - DOI - PMC - PubMed
-
- Banerjee RV, Johnston NL, Sobeski JK, Datta P, Matthews RG. Cloning and sequence analysis of the Escherichia coli metH gene encoding cobalamin-dependent methionine synthase and isolation of a tryptic fragment containing the cobalamin-binding domain. The Journal of Biological Chemistry. 1989;264:13888–13895. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

