Exportin Crm1 is repurposed as a docking protein to generate microtubule organizing centers at the nuclear pore
- PMID: 29809148
- PMCID: PMC6008054
- DOI: 10.7554/eLife.33465
Exportin Crm1 is repurposed as a docking protein to generate microtubule organizing centers at the nuclear pore
Abstract
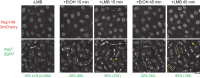
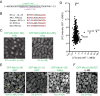
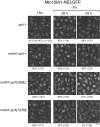
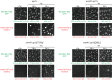
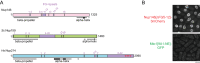
Non-centrosomal microtubule organizing centers (MTOCs) are important for microtubule organization in many cell types. In fission yeast Schizosaccharomyces pombe, the protein Mto1, together with partner protein Mto2 (Mto1/2 complex), recruits the γ-tubulin complex to multiple non-centrosomal MTOCs, including the nuclear envelope (NE). Here, we develop a comparative-interactome mass spectrometry approach to determine how Mto1 localizes to the NE. Surprisingly, we find that Mto1, a constitutively cytoplasmic protein, docks at nuclear pore complexes (NPCs), via interaction with exportin Crm1 and cytoplasmic FG-nucleoporin Nup146. Although Mto1 is not a nuclear export cargo, it binds Crm1 via a nuclear export signal-like sequence, and docking requires both Ran in the GTP-bound state and Nup146 FG repeats. In addition to determining the mechanism of MTOC formation at the NE, our results reveal a novel role for Crm1 and the nuclear export machinery in the stable docking of a cytoplasmic protein complex at NPCs.
Keywords: S. pombe; cell biology; exportin; microtubule; microtubule organizing center; nuclear pore.
© 2018, Bao et al.
Conflict of interest statement
XB, CS, TK, EL, JR, YH, TH, KS No competing interests declared
Figures






References
-
- Adachi Y, Yanagida M. Higher order chromosome structure is affected by cold-sensitive mutations in a Schizosaccharomyces pombe gene crm1+ which encodes a 115-kD protein preferentially localized in the nucleus and its periphery. The Journal of Cell Biology. 1989;108:1195–1207. doi: 10.1083/jcb.108.4.1195. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

