Structure of the CLC-1 chloride channel from Homo sapiens
- PMID: 29809153
- PMCID: PMC6019066
- DOI: 10.7554/eLife.36629
Structure of the CLC-1 chloride channel from Homo sapiens
Abstract
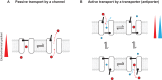
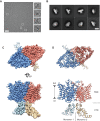
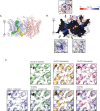
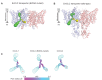
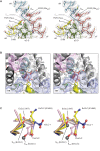
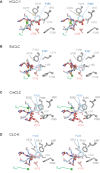
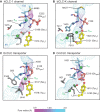
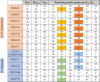
CLC channels mediate passive Cl- conduction, while CLC transporters mediate active Cl- transport coupled to H+ transport in the opposite direction. The distinction between CLC-0/1/2 channels and CLC transporters seems undetectable by amino acid sequence. To understand why they are different functionally we determined the structure of the human CLC-1 channel. Its 'glutamate gate' residue, known to mediate proton transfer in CLC transporters, adopts a location in the structure that appears to preclude it from its transport function. Furthermore, smaller side chains produce a wider pore near the intracellular surface, potentially reducing a kinetic barrier for Cl- conduction. When the corresponding residues are mutated in a transporter, it is converted to a channel. Finally, Cl- at key sites in the pore appear to interact with reduced affinity compared to transporters. Thus, subtle differences in glutamate gate conformation, internal pore diameter and Cl- affinity distinguish CLC channels and transporters.
Keywords: CLC; chloride channel; cryoelectron microscopy; human; molecular biophysics; structural biology.
© 2018, Park et al.
Conflict of interest statement
EP, RM No competing interests declared
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

