CO Oxidation by N2O Homogeneously Catalyzed by Ruthenium Hydride Pincer Complexes Indicating a New Mechanism
- PMID: 29812933
- PMCID: PMC6502446
- DOI: 10.1021/jacs.8b03927
CO Oxidation by N2O Homogeneously Catalyzed by Ruthenium Hydride Pincer Complexes Indicating a New Mechanism
Abstract
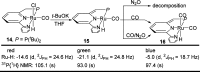
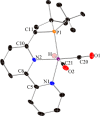
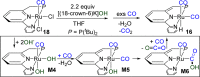
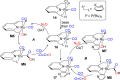
Both CO and N2O are important, environmentally harmful industrial gases. The reaction of CO and N2O to produce CO2 and N2 has stimulated much research interest aimed at degradation of these two gases in a single step. Herein, we report an efficient CO oxidation by N2O catalyzed by a (PNN)Ru-H pincer complex under mild conditions, even with no added base. The reaction is proposed to proceed through a sequence of O-atom transfer (OAT) from N2O to the Ru-H bond to form a Ru-OH intermediate, followed by intramolecular OH attack on an adjacent CO ligand, forming CO2 and N2. Thus, the Ru-H bond of the catalyst plays a central role in facilitating the OAT from N2O to CO, providing an efficient and novel protocol for CO oxidation.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
Similar articles
-
Non-innocent PNN ligand is important for CO oxidation by N2O catalyzed by a (PNN)Ru-H pincer complex: insights from DFT calculations.Dalton Trans. 2018 Nov 21;47(43):15324-15330. doi: 10.1039/c8dt03304h. Epub 2018 Oct 11. Dalton Trans. 2018. PMID: 30306993
-
Mechanism of the Facile Nitrous Oxide Fixation by Homogeneous Ruthenium Hydride Pincer Catalysts.Inorg Chem. 2020 Jul 6;59(13):9374-9383. doi: 10.1021/acs.inorgchem.0c01252. Epub 2020 Jun 23. Inorg Chem. 2020. PMID: 32574492
-
Oxidation of Alcohols to Carboxylates with N2O Catalyzed by Ruthenium(II)-CNC Complexes.ACS Catal. 2025 Jun 20;15(13):11530-11543. doi: 10.1021/acscatal.5c02021. eCollection 2025 Jul 4. ACS Catal. 2025. PMID: 40636731 Free PMC article.
-
Hydrogenation and Hydrosilylation of Nitrous Oxide Homogeneously Catalyzed by a Metal Complex.J Am Chem Soc. 2017 Apr 26;139(16):5720-5723. doi: 10.1021/jacs.7b02124. Epub 2017 Apr 12. J Am Chem Soc. 2017. PMID: 28402635 Free PMC article.
-
Mechanism of Alcohol-Water Dehydrogenative Coupling into Carboxylic Acid Using Milstein's Catalyst: A Detailed Investigation of the Outer-Sphere PES in the Reaction of Aldehydes with an Octahedral Ruthenium Hydroxide.Inorg Chem. 2016 Aug 15;55(16):7886-902. doi: 10.1021/acs.inorgchem.6b00766. Epub 2016 Jul 28. Inorg Chem. 2016. PMID: 27467132
Cited by
-
Catalytic Nitrous Oxide Reduction with H2 Mediated by Pincer Ir Complexes.Inorg Chem. 2022 Nov 21;61(46):18590-18600. doi: 10.1021/acs.inorgchem.2c02963. Epub 2022 Nov 8. Inorg Chem. 2022. PMID: 36346983 Free PMC article.
-
Catalytic Activation of N2O at a Low-Valent Bismuth Redox Platform.J Am Chem Soc. 2020 Nov 18;142(46):19473-19479. doi: 10.1021/jacs.0c10092. Epub 2020 Nov 4. J Am Chem Soc. 2020. PMID: 33146996 Free PMC article.
-
Bismuth Redox Catalysis: An Emerging Main-Group Platform for Organic Synthesis.ACS Catal. 2022 Jan 21;12(2):1382-1393. doi: 10.1021/acscatal.1c04897. Epub 2022 Jan 7. ACS Catal. 2022. PMID: 35096470 Free PMC article. Review.
-
Selective Carbanion-Pyridine Coordination of a Reactive P,N Ligand to RhI.Chemistry. 2019 Mar 12;25(15):3875-3883. doi: 10.1002/chem.201805504. Epub 2019 Feb 12. Chemistry. 2019. PMID: 30600857 Free PMC article.
-
MIL-100Cr with open Cr sites for a record N2O capture.Chem Commun (Camb). 2018 Dec 13;54(100):14061-14064. doi: 10.1039/c8cc07679k. Chem Commun (Camb). 2018. PMID: 30451265 Free PMC article.
References
-
- Prather M. Time Scales in Atmospheric Chemistry: Coupled Perturbations to N2O, NOy, and O3. Science 1998, 279, 1339–1341. 10.1126/science.279.5355.1339. - DOI - PubMed
- Ravishankara A. R.; Daniel J. S.; Portmann R. W. Nitrous Oxide (N2O): The Dominant Ozone-Depleting Substance Emitted in the 21st Century. Science 2009, 326, 123–125. 10.1126/science.1176985. - DOI - PubMed
- Highlight:Dameris M. Depletion of the Ozone Layer in the 21st Century. Angew. Chem., Int. Ed. 2010, 49, 489–491. 10.1002/anie.200906334. - DOI - PubMed
- U.S. Greenhouse Gas Inventory Report: 1990–2014, U.S. EPA; https://www.epa.gov/ghgemissions/us-greenhouse-gas-inventory-report-1990...
- Hansen J.; Sato M. Greenhouse gas growth rates. Proc. Natl. Acad. Sci. U. S. A. 2004, 101, 16109–16114. 10.1073/pnas.0406982101. - DOI - PMC - PubMed
-
-
For selected reviews on N2O chemistry, see:
- Tolman W. B. Binding and Activation of N2O at Transition-Metal Centers: Recent Mechanistic Insights. Angew. Chem., Int. Ed. 2010, 49, 1018–1024. 10.1002/anie.200905364. - DOI - PMC - PubMed
- Konsolakis M. Decomposition over Non-Noble-Metal Oxide Catalysts: Catalytic Performance, Mechanistic Considerations, and Surface Chemistry Aspects. ACS Catal. 2015, 5, 6397–6421. 10.1021/acscatal.5b01605. - DOI
- Severin K. Synthetic Chemistry with Nitrous Oxide. Chem. Soc. Rev. 2015, 44, 6375–6386. 10.1039/C5CS00339C. - DOI - PubMed
- Parmon V. N.; Panov G. I.; Uriarte A.; Noskov A. S. Nitrous oxide in oxidation chemistry and catalysis: application and production. Catal. Today 2005, 100, 115–131. 10.1016/j.cattod.2004.12.012. - DOI
- Pauleta S. R.; Dell’Acqua S.; Moura I. Nitrous oxide reductase. Coord. Chem. Rev. 2013, 257, 332–349. 10.1016/j.ccr.2012.05.026. - DOI
- Leont’ev A. V.; Fomicheva O. A.; Proskurnina M. V.; Zefirov N. S. Modern chemistry of nitrous oxide. Russ. Chem. Rev. 2001, 70, 91–104. 10.1070/RC2001v070n02ABEH000631. - DOI
- Lee D.-H.; Mondal B.; Karlin K. D.. Nitrogen Monoxide and Nitrous Oxide Binding and Reduction. In Activation of Small Molecules; Tolman W. B., Ed.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, 2006; pp 43–79.
-
-
- For a review on CO oxidation by N2O via single-atom catalysis, see:Schwarz H. Ménage-à-trois: Single-atom Catalysis, Mass Spectrometry, and Computational Chemistry. Catal. Sci. Technol. 2017, 7, 4302–4314. 10.1039/C6CY02658C. - DOI
-
-
For selected heterogeneous CO oxidation by N2O, see:
- McCabe R. W.; Wong C. Steady-State Kinetics of the CO-N2O Reaction over an Alumina-Supported Rhodium Catalyst. J. Catal. 1990, 121, 422–431. 10.1016/0021-9517(90)90250-N. - DOI
- Belton D. N.; Schmieg S. J. Kinetics of CO Oxidation by N2O over Rh(111). J. Catal. 1992, 138, 70–78. 10.1016/0021-9517(92)90007-5. - DOI
- Sadhankar R. R.; Ye J.; Lynch D. T. N2O Reducation by CO over an Alumina-Supported Pt Catalyst: Steady-State Multiplicity. J. Catal. 1994, 146, 511–522. 10.1006/jcat.1994.1089. - DOI
- Gluhoi A. C.; Dekkers M. A. P.; Nieuwenhuys B. E. Comparative Studies of The N2O/H2, N2O/CO, H2/O2 and CO/O2 Reactions on Supported Gold Catalysts: Effect of the Addition of Various Oxides. J. Catal. 2003, 219, 197–205. 10.1016/S0021-9517(03)00185-4. - DOI
- Zhdanov V. P.; Ma Y.; Matsushima T. Analysis of the Kinetics of N2O-CO reaction on Pd(110). Surf. Sci. 2005, 583, 36–45. 10.1016/j.susc.2005.03.020. - DOI
- Zhdanov V. P.; Nakagoe O.; Matsushima T. Kinetics of the N2O-CO reaction on Rh(110). Surf. Sci. 2007, 601, L49–L54. 10.1016/j.susc.2007.03.019. - DOI
- Jia A.-P.; Jiang S.-Y.; Lu J.-Q.; Luo M.-F. Study of Catalytic Activity at the CuO-CeO2 Interface for CO oxidation. J. Phys. Chem. C 2010, 114, 21605–21610. 10.1021/jp108556u. - DOI
-
-
-
For stoichiometric CO oxidation by N2O in solution, see:
- Bottomley F.; Lin I. J. B.; Mukaida M. Reactions of Dinitrogen Oxide (Nitrous Oxide) with Dicyclopentadienyltitanium Complexes Including a Reaction in Which Carbon Monoxide Is Oxidized. Carbon Monoxide-Induced N–N Bond Cleavage of Nitrous Oxide That Is Competitive with Oxygen Atom Transfer to Carbon Monoxide as Mediated by a Mo(II)/Mo(IV) Catalytic Cycle. J. Am. Chem. Soc. 1980, 102, 5238–5242. 10.1021/ja00536a020. - DOI - PubMed
- Reeds J. P.; Yonke B. L.; Zavalij P. Y.; Sita L. R. Carbon Monoxide-Induced N–N Bond Cleavage of Nitrous Oxide That Is Competitive with Oxygen Atom Transfer to Carbon Monoxide as Mediated by a Mo(II)/Mo(IV) Catalytic Cycle. J. Am. Chem. Soc. 2011, 133, 18602–18605. 10.1021/ja208669s. - DOI - PubMed
- Yonke B. L.; Reeds J. P.; Zavalij P. Y.; Sita L. R. Catalytic Degenerate and Nondegenerate Oxygen Atom Transfers Employing N2O and CO2 and a MII/MIV Cycle Mediated by Group 6 MIV Terminal Oxo Complexes. Angew. Chem., Int. Ed. 2011, 50, 12342–12346. 10.1002/anie.201106074. - DOI - PubMed
- Xie H.; Yang L.; Ye X.; Cao Z. Mechanism of Carbon Monoxide Induced N–N Bond Cleavage of Nitrous Oxide Mediated by Molybdenum Complexes: A DFT Study. Organometallics 2014, 33, 1553–1562. 10.1021/om400935f. - DOI
-
A related paper:
- Horn B.; Limberg C.; Herwig C.; Feist M.; Mebs S. CO Oxidation at Nickel Centres by N2O or O2 to Yield a Novel Hexanuclear Carbonate. Chem. Commun. 2012, 48, 8243–8245. 10.1039/c2cc33846g. - DOI - PubMed
-
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

