Detecting change in stochastic sound sequences
- PMID: 29813049
- PMCID: PMC5993325
- DOI: 10.1371/journal.pcbi.1006162
Detecting change in stochastic sound sequences
Abstract
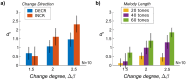
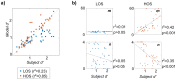
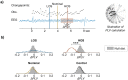
Our ability to parse our acoustic environment relies on the brain's capacity to extract statistical regularities from surrounding sounds. Previous work in regularity extraction has predominantly focused on the brain's sensitivity to predictable patterns in sound sequences. However, natural sound environments are rarely completely predictable, often containing some level of randomness, yet the brain is able to effectively interpret its surroundings by extracting useful information from stochastic sounds. It has been previously shown that the brain is sensitive to the marginal lower-order statistics of sound sequences (i.e., mean and variance). In this work, we investigate the brain's sensitivity to higher-order statistics describing temporal dependencies between sound events through a series of change detection experiments, where listeners are asked to detect changes in randomness in the pitch of tone sequences. Behavioral data indicate listeners collect statistical estimates to process incoming sounds, and a perceptual model based on Bayesian inference shows a capacity in the brain to track higher-order statistics. Further analysis of individual subjects' behavior indicates an important role of perceptual constraints in listeners' ability to track these sensory statistics with high fidelity. In addition, the inference model facilitates analysis of neural electroencephalography (EEG) responses, anchoring the analysis relative to the statistics of each stochastic stimulus. This reveals both a deviance response and a change-related disruption in phase of the stimulus-locked response that follow the higher-order statistics. These results shed light on the brain's ability to process stochastic sound sequences.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




References
-
- Bendixen A. Predictability effects in auditory scene analysis: a review. Frontiers in Neuroscience. 2014;8:60 doi: 10.3389/fnins.2014.00060 - DOI - PMC - PubMed
-
- Winkler I, Denham SL, Nelken I. Modeling the auditory scene: predictive regularity representations and perceptual objects. Trends in Cognitive Sciences. 2009;13(12):532–540. doi: 10.1016/j.tics.2009.09.003 - DOI - PubMed
-
- Bendixen A, Schröger E, Ritter W, Winkler I. Regularity extraction from non-adjacent sounds. Frontiers in Psychology. 2012;3 doi: 10.3389/fpsyg.2012.00143 - DOI - PMC - PubMed
-
- Saarinen J, Paavilainen P, Schöger E, Tervaniemi M, Näätänen R. Representation of abstract attributes of auditory stimuli in the human brain. NeuroReport. 1992;3(12):1149–51. doi: 10.1097/00001756-199212000-00030 - DOI - PubMed
-
- Paavilainen P, Degerman A, Takegata R, Winkler I. Spectral and temporal stimulus characteristics in the processing of abstract auditory features. Neuroreport. 2003;14(5):715–8. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

