Association Between Vitamin D Supplementation During Pregnancy and Offspring Growth, Morbidity, and Mortality: A Systematic Review and Meta-analysis
- PMID: 29813153
- PMCID: PMC6137512
- DOI: 10.1001/jamapediatrics.2018.0302
Association Between Vitamin D Supplementation During Pregnancy and Offspring Growth, Morbidity, and Mortality: A Systematic Review and Meta-analysis
Abstract
Importance: Whether vitamin D supplementation during pregnancy is beneficial and safe for offspring is unclear.
Objective: To systematically review studies of the effects of vitamin D supplementation during pregnancy on offspring growth, morbidity, and mortality.
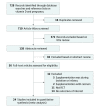
Data sources: Searches of Medline, Embase, and the Cochrane Database of Systematic Reviews were conducted up to October 31, 2017. Key search terms were vitamin D, pregnancy, randomized controlled trials, and offspring outcomes.
Study selection: Randomized clinical trials of vitamin D supplementation during pregnancy and offspring outcomes.
Data extraction and synthesis: Two authors independently extracted data, and the quality of the studies was assessed. Summary risk ratio (RR), risk difference (RD) or mean difference (MD), and 95% CI were calculated using fixed-effects or random-effects meta-analysis.
Main outcomes and measures: Main outcomes were fetal or neonatal mortality, small for gestational age (SGA), congenital malformation, admission to a neonatal intensive care unit, birth weight, Apgar scores, neonatal 25-hydroxyvitamin D (25[OH]D) and calcium concentrations, gestational age, preterm birth, infant anthropometry, and respiratory morbidity during childhood.
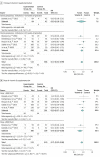
Results: Twenty-four clinical trials involving 5405 participants met inclusion criteria. Vitamin D supplementation during pregnancy was associated with a lower risk of SGA (RR, 0.72; 95% CI, 0.52 to 0.99; RD, -5.60%; 95% CI, -0.86% to -10.34%) without risk of fetal or neonatal mortality (RR, 0.72; 95% CI, 0.47 to 1.11) or congenital abnormality (RR, 0.94; 95% CI, 0.61 to 1.43). Neonates with prenatal vitamin D supplementation had higher 25(OH)D levels (MD, 13.50 ng/mL; 95% CI, 10.12 to 16.87 ng/mL), calcium levels (MD, 0.19 mg/dL; 95% CI, 0.003 to 0.38 mg/dL), and weight at birth (MD, 75.38 g; 95% CI, 22.88 to 127.88 g), 3 months (MD, 0.21 kg; 95% CI, 0.13 to 0.28 kg), 6 months (MD, 0.46 kg; 95% CI, 0.33 to 0.58 kg), 9 months (MD, 0.50 kg; 95% CI, 0.01 to 0.99 kg), and 12 months (MD, 0.32 kg; 95% CI, 0.12 to 0.52 kg). Subgroup analysis by doses showed that low-dose vitamin D supplementation (≤2000 IU/d) was associated with a reduced risk of fetal or neonatal mortality (RR, 0.35; 95% CI, 0.15 to 0.80), but higher doses (>2000 IU/d) did not reduce this risk (RR, 0.95; 95% CI, 0.59 to 1.54).
Conclusions and relevance: Vitamin D supplementation during pregnancy is associated with a reduced risk of SGA and improved infant growth without risk of fetal or neonatal mortality or congenital abnormality. Vitamin D supplementation with doses of 2000 IU/d or lower during pregnancy may reduce the risk of fetal or neonatal mortality.
Conflict of interest statement
Figures

Comment in
-
Prenatal Vitamin D Supplementation to Improve Health in Offspring.JAMA Pediatr. 2018 Jul 1;172(7):617-618. doi: 10.1001/jamapediatrics.2018.0917. JAMA Pediatr. 2018. PMID: 29813164 No abstract available.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

