Functional Role of Non-Coding RNAs during Epithelial-To-Mesenchymal Transition
- PMID: 29843425
- PMCID: PMC6027143
- DOI: 10.3390/ncrna4020014
Functional Role of Non-Coding RNAs during Epithelial-To-Mesenchymal Transition
Abstract
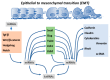
Epithelial-to-mesenchymal transition (EMT) is a key biological process involved in a multitude of developmental and pathological events. It is characterized by the progressive loss of cell-to-cell contacts and actin cytoskeletal rearrangements, leading to filopodia formation and the progressive up-regulation of a mesenchymal gene expression pattern enabling cell migration. Epithelial-to-mesenchymal transition is already observed in early embryonic stages such as gastrulation, when the epiblast undergoes an EMT process and therefore leads to the formation of the third embryonic layer, the mesoderm. Epithelial-to-mesenchymal transition is pivotal in multiple embryonic processes, such as for example during cardiovascular system development, as valve primordia are formed and the cardiac jelly is progressively invaded by endocardium-derived mesenchyme or as the external cardiac cell layer is established, i.e., the epicardium and cells detached migrate into the embryonic myocardial to form the cardiac fibrous skeleton and the coronary vasculature. Strikingly, the most important biological event in which EMT is pivotal is cancer development and metastasis. Over the last years, understanding of the transcriptional regulatory networks involved in EMT has greatly advanced. Several transcriptional factors such as Snail, Slug, Twist, Zeb1 and Zeb2 have been reported to play fundamental roles in EMT, leading in most cases to transcriptional repression of cell⁻cell interacting proteins such as ZO-1 and cadherins and activation of cytoskeletal markers such as vimentin. In recent years, a fundamental role for non-coding RNAs, particularly microRNAs and more recently long non-coding RNAs, has been identified in normal tissue development and homeostasis as well as in several oncogenic processes. In this study, we will provide a state-of-the-art review of the functional roles of non-coding RNAs, particularly microRNAs, in epithelial-to-mesenchymal transition in both developmental and pathological EMT.
Keywords: epithelial-to-mesenchymal transition; lncRNAs; microRNA; post-transcriptional regulation; transcriptional regulation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Patel N., Garikapati K.R., Makani V.K.K., Nair A.D., Vangara N., Bhadra U., Pal Bhadra M. Regulating BMI1 expression via miRNAs promote Mesenchymal to Epithelial Transition (MET) and sensitizes breast cancer cell to chemotherapeutic drug. PLoS ONE. 2018;13:e0190245. doi: 10.1371/journal.pone.0190245. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

