Zinc Metallochaperones as Mutant p53 Reactivators: A New Paradigm in Cancer Therapeutics
- PMID: 29843463
- PMCID: PMC6025018
- DOI: 10.3390/cancers10060166
Zinc Metallochaperones as Mutant p53 Reactivators: A New Paradigm in Cancer Therapeutics
Abstract
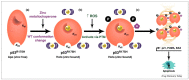
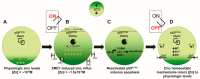
Restoration of wild-type structure and function to mutant p53 with a small molecule (hereafter referred to as "reactivating" mutant p53) is one of the holy grails in cancer therapeutics. The majority of TP53 mutations are missense which generate a defective protein that is targetable. We are currently developing a new class of mutant p53 reactivators called zinc metallochaperones (ZMCs) and, here, we review our current understanding of them. The p53 protein requires the binding of a single zinc ion, coordinated by four amino acids in the DNA binding domain, for proper structure and function. Loss of the wild-type structure by impairing zinc binding is a common mechanism of inactivating p53. ZMCs reactivate mutant p53 using a novel two-part mechanism that involves restoring the wild-type structure by reestablishing zinc binding and activating p53 through post-translational modifications induced by cellular reactive oxygen species (ROS). The former causes a wild-type conformation change, the later induces a p53-mediated apoptotic program to kill the cancer cell. ZMCs are small molecule metal ion chelators that bind zinc and other divalent metal ions strong enough to remove zinc from serum albumin, but weak enough to donate it to mutant p53. Recently we have extended our understanding of the mechanism of ZMCs to the role of cells' response to this zinc surge. We found that cellular zinc homeostatic mechanisms, which normally function to maintain free intracellular zinc levels in the picomolar range, are induced by ZMCs. By normalizing zinc levels, they function as an OFF switch to ZMCs because zinc levels are no longer sufficiently high to maintain a wild-type structure. This on/off switch leads to a transient nature to the mechanism of ZMCs in which mutant p53 activity comes on in a few hours and then is turned off. This finding has important implications for the translation of ZMCs to the clinic because it indicates that ZMC concentrations need not be maintained at high levels for their activity. Indeed, we found that short exposures (as little as 15 min) were adequate to observe the mutant p53 reactivating activity. This switch mechanism imparts an advantage over other targeted therapeutics in that efficacy can be accomplished with minimal exposure which minimizes toxicity and maximizes the therapeutic window. This on/off switch mechanism is unique in targeted cancer therapeutics and will impact the design of human clinical trials.
Keywords: mutant p53; on/off switch mechanism; pancreatic cancer; zinc homeostasis; zinc metallochaperones.
Conflict of interest statement
Samuel Kogan declares no conflict of interest, Darren Carpizo is the founder of Z53 Therapeutics, Inc.
Figures
Similar articles
-
Zinc Metallochaperones Reactivate Mutant p53 Using an ON/OFF Switch Mechanism: A New Paradigm in Cancer Therapeutics.Clin Cancer Res. 2018 Sep 15;24(18):4505-4517. doi: 10.1158/1078-0432.CCR-18-0822. Epub 2018 Jun 18. Clin Cancer Res. 2018. PMID: 29914895 Free PMC article.
-
Thiosemicarbazones Functioning as Zinc Metallochaperones to Reactivate Mutant p53.Mol Pharmacol. 2017 Jun;91(6):567-575. doi: 10.1124/mol.116.107409. Epub 2017 Mar 20. Mol Pharmacol. 2017. PMID: 28320780 Free PMC article.
-
Combinatorial Therapy of Zinc Metallochaperones with Mutant p53 Reactivation and Diminished Copper Binding.Mol Cancer Ther. 2019 Aug;18(8):1355-1365. doi: 10.1158/1535-7163.MCT-18-1080. Epub 2019 Jun 13. Mol Cancer Ther. 2019. PMID: 31196889 Free PMC article.
-
Reactivating mutant p53 using small molecules as zinc metallochaperones: awakening a sleeping giant in cancer.Drug Discov Today. 2015 Nov;20(11):1391-7. doi: 10.1016/j.drudis.2015.07.006. Epub 2015 Jul 20. Drug Discov Today. 2015. PMID: 26205328 Free PMC article. Review.
-
p53 and Zinc: A Malleable Relationship.Front Mol Biosci. 2022 Apr 13;9:895887. doi: 10.3389/fmolb.2022.895887. eCollection 2022. Front Mol Biosci. 2022. PMID: 35495631 Free PMC article. Review.
Cited by
-
The Impact of Mutant p53 in the Non-Coding RNA World.Biomolecules. 2020 Mar 19;10(3):472. doi: 10.3390/biom10030472. Biomolecules. 2020. PMID: 32204575 Free PMC article. Review.
-
High-resolution melting effectively pre-screens for TP53 mutations before direct sequencing in patients with diffuse glioma.Hum Cell. 2021 Mar;34(2):644-653. doi: 10.1007/s13577-020-00471-2. Epub 2021 Jan 17. Hum Cell. 2021. PMID: 33454902
-
Discovery of Nanomolar-Affinity Pharmacological Chaperones Stabilizing the Oncogenic p53 Mutant Y220C.ACS Pharmacol Transl Sci. 2022 Oct 11;5(11):1169-1180. doi: 10.1021/acsptsci.2c00164. eCollection 2022 Nov 11. ACS Pharmacol Transl Sci. 2022. PMID: 36407959 Free PMC article.
-
The pleiotropic role of p53 in functional/dysfunctional neurons: focus on pathogenesis and diagnosis of Alzheimer's disease.Alzheimers Res Ther. 2020 Dec 3;12(1):160. doi: 10.1186/s13195-020-00732-0. Alzheimers Res Ther. 2020. PMID: 33272326 Free PMC article. Review.
-
Helping the Released Guardian: Drug Combinations for Supporting the Anticancer Activity of HDM2 (MDM2) Antagonists.Cancers (Basel). 2019 Jul 19;11(7):1014. doi: 10.3390/cancers11071014. Cancers (Basel). 2019. PMID: 31331108 Free PMC article. Review.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

