Risk factors for peripartum hysterectomy among women with postpartum haemorrhage: analysis of data from the WOMAN trial
- PMID: 29843627
- PMCID: PMC5975404
- DOI: 10.1186/s12884-018-1829-7
Risk factors for peripartum hysterectomy among women with postpartum haemorrhage: analysis of data from the WOMAN trial
Abstract
Background: Peripartum hysterectomy can cause significant morbidity and mortality. Most studies of peripartum hysterectomy are from high income countries. This cohort study examined risk factors for peripartum hysterectomy using data from Africa, Asia, Europe and the Americas.
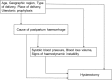
Methods: We used data from the World Maternal Antifibrinolytic (WOMAN) trial carried out in 193 hospitals in 21 countries. Peripartum hysterectomy was defined as hysterectomy within 6 weeks of delivery as a complication of postpartum haemorrhage. Univariable and multivariable random effects logistic regression models were used to analyse risk factors. A hierarchical conceptual framework guided our multivariable analysis.
Results: Five percent of women had a hysterectomy (1020/20,017). Haemorrhage from placenta praevia/accreta carried a higher risk of hysterectomy (17%) than surgical trauma/tears (5%) and uterine atony (3%). The adjusted odds ratio (AOR) for hysterectomy in women with placenta praevia/accreta was 3.2 (95% CI: 2.7-3.8), compared to uterine atony. The risk of hysterectomy increased with maternal age. Caesarean section was associated with fourfold higher odds of hysterectomy than vaginal delivery (AOR 4.3, 95% CI: 3.6-5.0). Mothers in Asia had a higher hysterectomy incidence (7%) than mothers in Africa (5%) (AOR: 1.2, 95% CI: 0.9-1.7).
Conclusions: Placenta praevia/accreta is associated with a higher risk of peripartum hysterectomy. Other risk factors for hysterectomy are advanced maternal age, caesarean section and giving birth in Asia.
Keywords: Africa; Asia; Caesarean section; Conceptual framework; Peripartum hysterectomy; Placenta accreta; Postpartum haemorrhage.
Conflict of interest statement
Ethics approval and consent to participate
This is a secondary analysis of the WOMAN trial data. The need for ethics approval and consent was waived as per national guidelines. The relevant ethics committees and regulatory agencies approved the consent procedures at each trial site for the original study. We obtained informed consent from women if their physical and mental capacity allowed. If a woman could not give consent, we obtained proxy consent from a relative or representative. If no proxy was available, then if local regulation allowed, we deferred or waived the consent. In these cases, we told the woman about the trial as soon as possible and obtained consent for use of the data collected. The consent procedures are described in detail in the Woman trial protocol.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical