Predictors of medication adherence among patients with severe psychiatric disorders: findings from the baseline assessment of a randomized controlled trial (Tecla)
- PMID: 29843676
- PMCID: PMC5975380
- DOI: 10.1186/s12888-018-1737-4
Predictors of medication adherence among patients with severe psychiatric disorders: findings from the baseline assessment of a randomized controlled trial (Tecla)
Abstract
Background: Schizophrenia and bipolar disorder are characterized by a high disease burden. Antipsychotic medication is an essential part of the treatment. However, non-adherence is a major problem. Our aim was to examine potential determinants of non-adherence for patients with severe mental disorders.
Methods: Baseline data of the study "Post stationary telemedical care of patients with severe psychiatric disorders" (Tecla) were used. Medication adherence was assessed with the Medication Adherence Report Scale German version (MARS-D). A logistic regression was calculated with age, sex, education, employment status, level of global functioning, social support and intake of typical and atypical antipsychotics as predictors.
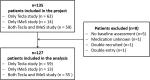
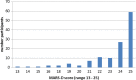
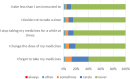
Results: N = 127 participants were included in the analysis (n = 73 men, mean age 42 years). The mean MARS-D Score was 23.4 (SD 2.5). The most common reason for non-adherence was forgetting to take the medicine. Significant positive determinants for adherence were older age (OR 1.02, 95% CI 1.011-1.024, p < 0.0001), being employed (OR 2.46, 95% CI 1.893-3.206, p < 0.0001), higher level of global functioning (overall measure of how patients are doing) (OR 1.02, 95% CI 1.012-1.028, p < 0.0001), having social support (OR 1.02, 95% CI 1.013-1.026, p < 0.0001), and intake of typical antipsychotics (OR 2.389, 95% CI 1.796-3.178, p < 0.0001). A negative determinant was (female) sex (OR 0.73, 95% CI 0.625-0.859, p = 0.0001).
Conclusions: Especially employment, functioning and social support could be promising targets to facilitate adherence in patients with schizophrenia or bipolar disorder.
Trial registration: This study is retrospectively registered at the German Clinical Trials Register with the trial registration number DRKS00008548 at 21/05/2015.
Keywords: Adherence; Bipolar disorders; MARS-D; Mental health disorders; Non-adherence; Psychiatry; Psychotic disorders; Schizophrenia.
Conflict of interest statement
Ethics approval and consent to participate
Tecla is approved by the Ethics Committee of the University Medicine Greifswald (BB 122/14). The committee stated that the majority of the members of the committee concluded that there are no ethical and legal concerns against the implementation of the study, and therefore approves the proposal. Tecla is retrospectively registered at 2015\05\21 at the German Clinical Trials Register (DRKS00008548). IMeS is also approved by the Ethics Committee of the University Medicine Greifswald; BB 017/15. All patients had to sign an informed consent to participate. All appropriate legal guardians or representatives were informed about the participation. All guardians or representatives indicated that the patients were capable of providing ethical consent to participate.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
-
- Jacobi F, Höfler M, Strehle J, Mack S, Gerschler A, Scholl L, Busch MA, Maske U, Hapke U, Gaebel W, et al. Nervenarzt. 2014. Mental disorders in the general population. Study on the health of adults in Germany and the additional module mental health (DEGS1-MH) pp. 77–87. - PubMed
-
- Gaebel W, Wölwer W. Schizophrenie. Berlin: Robert Koch-Institut; 2010.
-
- Loebel A, Lieberman J, Alvir J. Time to treatment response in successive episodes of early onset schizophrenia. Schizophrenia. 1995;1–2:158. doi: 10.1016/0920-9964(95)95489-V. - DOI
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

