Therapeutic effects of adipose-tissue-derived mesenchymal stromal cells and their extracellular vesicles in experimental silicosis
- PMID: 29843724
- PMCID: PMC5975461
- DOI: 10.1186/s12931-018-0802-3
Therapeutic effects of adipose-tissue-derived mesenchymal stromal cells and their extracellular vesicles in experimental silicosis
Abstract
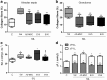
Background: Silicosis is an occupational disease that affects workers who inhale silica particles, leading to extensive lung fibrosis and ultimately causing respiratory failure. Mesenchymal stromal cells (MSCs) have been shown to exert therapeutic effects in lung diseases and represent an alternative treatment for silicosis. Recently, it has been suggested that similar effects can be achieved by the therapeutic use of extracellular vesicles (EVs) obtained from MSCs. The aim of this study was to investigate the effects of adipose-tissue-derived MSCs (AD-MSCs) or their EVs in a model of silicosis.
Methods: Silicosis was induced by intratracheal instillation of silica in C57BL/6 mice. After the onset of disease, animals received saline, AD-MSCs, or EVs, intratracheally.
Results: At day 30, AD-MSCs and EVs led to a reduction in collagen fiber content, size of granuloma, and in the number of macrophages inside granuloma and in the alveolar septa. In addition, the expression levels of interleukin 1β and transforming growth factor beta in the lungs were decreased. Higher dose of EVs also reduced lung static elastance when compared with the untreated silicosis group.
Conclusions: Both AD-MSCs and EVs, locally delivered, ameliorated fibrosis and inflammation, but dose-enhanced EVs yielded better therapeutic outcomes in this model of silicosis.
Keywords: Extracellular vesicles; Fibrosis; Inflammation; Mesenchymal stromal cells; Silicosis.
Conflict of interest statement
Ethics approval
This study was approved by the Health Sciences Ethics Committee of the Federal University of Rio de Janeiro (CEUA 188/13). All animals received humane care in compliance with the principles of laboratory animal care formulated by the National Society of Medical Research and the Guide for the Care and Use of Laboratory Animals prepared by the National Academy of Sciences, USA.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures






References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

