miR-519a enhances chemosensitivity and promotes autophagy in glioblastoma by targeting STAT3/Bcl2 signaling pathway
- PMID: 29843746
- PMCID: PMC5975545
- DOI: 10.1186/s13045-018-0618-0
miR-519a enhances chemosensitivity and promotes autophagy in glioblastoma by targeting STAT3/Bcl2 signaling pathway
Abstract
Background: Chemoresistance to temozolomide (TMZ) is a major challenge in the treatment of glioblastoma (GBM). We previously found that miR-519a functions as a tumor suppressor in glioma by targeting the signal transducer and activator of transcription 3 (STAT3)-mediated autophagy oncogenic pathway. Here, we investigated the effects of miR-519a on TMZ chemosensitivity and autophagy in GBM cells. Furthermore, the underlying molecular mechanisms and signaling pathways were explored.
Methods: In the present study, two stable TMZ-resistant GBM cell lines were successfully generated by exposure of parental cells to a gradually increasing TMZ concentration. After transfecting U87-MG/TMZ and U87-MG cells with miR-519a mimic or inhibitor, a series of biochemical assays such as MTT, apoptosis, and colony formation were performed to determine the chemosensitive response to TMZ. The autophagy levels in GBM cells were detected by transmission electron microscopy, LC3B protein immunofluorescence, and Western blotting analysis. Stable knockdown and overexpression of miR-519a in GBM cells were established using lentivirus. A xenograft nude mouse model and in situ brain model were used to examine the in vivo effects of miR-519a. Tumor tissue samples were collected from 48 patients with GBM and were used to assess the relationship between miR-519a and STAT3 expression.
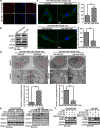
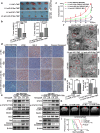
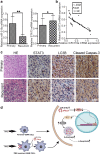
Results: TMZ treatment significantly upregulated miR-519a in U87-MG cells but not in U87-MG/TMZ cells. Moreover, the expression of miR-519a and baseline autophagy levels was lower in U87-MG/TMZ cells as compared to U87-MG cells. miR-519a dramatically enhanced TMZ-induced autophagy and apoptotic cell death in U87-MG/TMZ cells, while inhibition of miR-519a promoted TMZ resistance and reduced TMZ-induced autophagy in U87-MG cells. Furthermore, miR-519a induced autophagy through modification of STAT3 expression. The in vivo results showed that miR-519a can enhance apoptosis and sensitized GBM to TMZ treatment by promoting autophagy and targeting the STAT3/Bcl-2/Beclin-1 pathway. In human GBM tissues, we found an inverse correlation between miR-519a and STAT3 expression.
Conclusions: Our results suggested that miR-519a increased the sensitivity of GBM cells to TMZ therapy. The positive effects of miR-519a may be mediated through autophagy. In addition, miR-519a overexpression can induce autophagy by inhibiting STAT3/Bcl-2 pathway. Therefore, a combination of miR-519a and TMZ may represent an effective therapeutic strategy in GBM.
Keywords: Autophagy; Chemoresistance; Glioblastoma; Signal transducer and activator of transcription 3; miR-519a.
Conflict of interest statement
Ethics approval and consent to participate
This study was approved by the Ethics Committee of Nanfang Hospital. Written informed consent was obtained from all subjects, adhered to the Declaration of Helsinki.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

