Torque teno virus dynamics during the first year of life
- PMID: 29843750
- PMCID: PMC5975406
- DOI: 10.1186/s12985-018-1007-6
Torque teno virus dynamics during the first year of life
Abstract
Background: Torque teno virus is a small chronically persisting circular negative ssDNA virus reaching near 100% prevalence. It is reported to be a marker for immune function in immunocompromised patients. The possibility of vertical maternal-fetal transmission remains controversial but incidence rate of TTV DNA in children increased with age. TTV dynamics well studied for allogeneic hematopoietic stem cell transplantation as a predictor of post-transplant complications but there is no viral proliferation kinetics data for other patient groups or healthy individuals. The aim of this study was to determine TTV dynamics during the first year of life of healthy infants.
Methods: Ninety eight clinically healthy breastfeeding infants (1-12 months of age) were analyzed by quantitative PCR for the whole blood TTV load with the test sensitivity of about 1000 viral copies per milliliter of blood (total number of samples including repeatedly tested infants was 109).
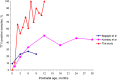
Results: 67% of all analyzed samples were TTV-positive demonstrating significant positive correlation between age and TTV load (r = 0.81, p < 0.01).
Conclusions: This is the first study to suggest that viral load increases during the first year of life reaching a plateau after 6 months with strong proliferation for the first 60 days. Our data well correlates with TTV dynamics in patients following allogeneic hematopoietic stem cell transplantation.
Keywords: Infants; Neonatal period; TORCH infections; TTV; Torque teno virus; Transfusion-transmitted virus; Viral load dynamics.
Conflict of interest statement
Ethics approval and consent to participate
The study protocol was reviewed and approved by the Ethics Committee of the Pirogov Russian National Research Medical University (Protocol No.2017/23); the study was conducted in accordance with the Declaration of Helsinki. All participants (children’s parents) provided written informed consent.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
-
- AbuOdeh R, Al-Mawlawi N, Al-Qahtani AA, Bohol MF, Al-Ahdal MN, Hasan HA, AbuOdeh L, Nasrallah GK. Detection and genotyping of torque teno virus (TTV) in healthy blood donors and patients infected with HBV or HCV in Qatar. J Med Virol. 2015; 10.1002/jmv.24146. - PubMed
-
- Bostan N, Nabgha E Amen, Bokhari H. Current and Future Prospects of Torque Teno Virus. J Vaccines Vaccin. 2013. doi:10.4172/2157-7560.S1-004
-
- Maggi F, Pifferi M, Tempestini E, Fornai C, Lanini L, Andreoli E, Vatterloni M, Presciuttini S, Pietrobelli A, Boner A, Pistello M, Bendinelli M. TT virus load and lymphocyte subpopulations in children with acute respiratory diseases. J Virol. 2003;77:9081–9083. doi: 10.1128/JVI.77.16.9081-9083.2003. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources