Stereotactic body radiation therapy (SBRT) in patients with hepatocellular carcinoma and oligometastatic liver disease
- PMID: 29843752
- PMCID: PMC5975506
- DOI: 10.1186/s13014-018-1048-4
Stereotactic body radiation therapy (SBRT) in patients with hepatocellular carcinoma and oligometastatic liver disease
Abstract
Background: To report our experience with SBRT in primary and secondary liver tumors.
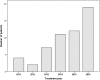
Methods: We retrospectively analysed 55 patients (70 lesions) with a median follow-up of 10 months (range 1-57) treated from 2011 to 2016. All patients had not been eligible for other local treatment options. Median age was 64 years and 64% were male. 27 patients (36 lesions) suffered from hepatocellular carcinoma (HCC, Child A:78%, Child B:18%, Child C:4%), 28 patients (34 lesions) had oligometastatic liver disease (MD). Treatment planning was based on 4D-CT usually after placement of fiducials. Dose and fractionation varied depending on localization and size, most commonly 3 × 12.5 Gy (prescribed to the surrounding 65%-isodose) in 56% and 5x8Gy (80% isodose) in 20% of the treated lesions.
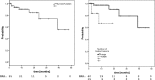
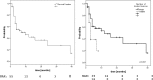
Results: Local recurrence was observed in 7 patients (13%) and 8 lesions (11%), resulting in estimated 1- and 2-year local control rates (LC) of 91 and 74%. Estimated 1- and 2-year rates of Freedom from hepatic failure (FFHF) were 42 and 28%. Number of lesions was predictive for LC and FFHF in the entire cohort. Estimated 1- and 2-year overall survival (OS) was 76 and 57%. OS was significantly affected by number of treated lesions and performance status. In the HCC subgroup, pretreatment liver function and gender were also predictive for OS. Maximum acute non-hepatic toxicity was grade 1 in 16% and grade 2 in 10% of the patients. Three HCC patients (11%) developed marked deterioration of liver function (grade 3/4).
Conclusions: SBRT resulted in high local control and acceptable survival rates in patients with HCC or MD not amendable to other locally-ablative treatment options with limited toxicity. Care should be taken in HCC patients with Child B cirrhosis.
Keywords: HCC; Liver; Oligometastatic; SBRT.
Conflict of interest statement
Ethics approval and consent to participate
The study was approved by the Ethics committee of the University of Munich (LMU), reference number 617–16.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures

References
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

