Oxidative stress impairs energy metabolism in primary cells and synovial tissue of patients with rheumatoid arthritis
- PMID: 29843785
- PMCID: PMC5972404
- DOI: 10.1186/s13075-018-1592-1
Oxidative stress impairs energy metabolism in primary cells and synovial tissue of patients with rheumatoid arthritis
Abstract
Background: In this study, we examined the effect of oxidative stress on cellular energy metabolism and pro-angiogenic/pro-inflammatory mechanisms of primary rheumatoid arthritis synovial fibroblast cells (RASFC) and human umbilical vein endothelial cells (HUVEC).
Methods: Primary RASFC and HUVEC were cultured with the oxidative stress inducer 4-hydroxy-2-nonenal (4-HNE), and extracellular acidification rate, oxygen consumption rate, mitochondrial function and pro-angiogenic/pro-inflammatory mechanisms were assessed using the Seahorse analyser, complex I-V activity assays, random mutation mitochondrial capture assays, enzyme-linked immunosorbent assays and functional assays, including angiogenic tube formation, migration and invasion. Expression of angiogenic growth factors in synovial tissue (ST) was assessed by IHC in patients with rheumatoid arthritis (RA) undergoing arthroscopy before and after administration of tumour necrosis factor inhibitors (TNFi).
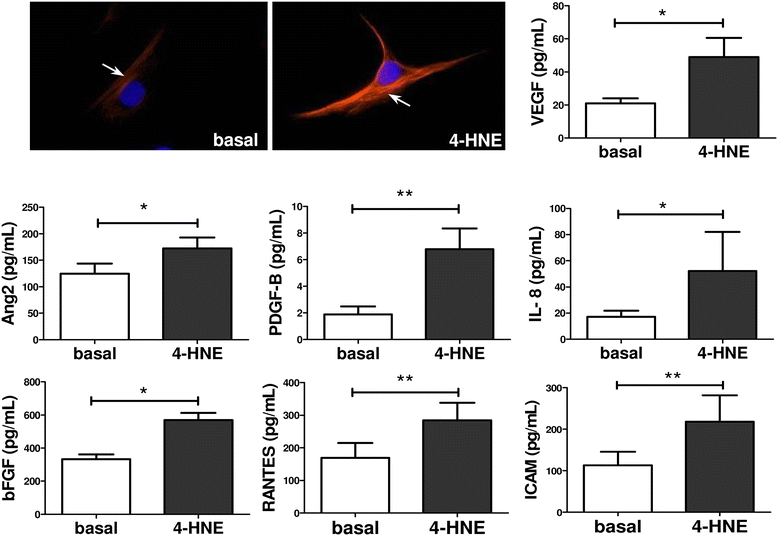
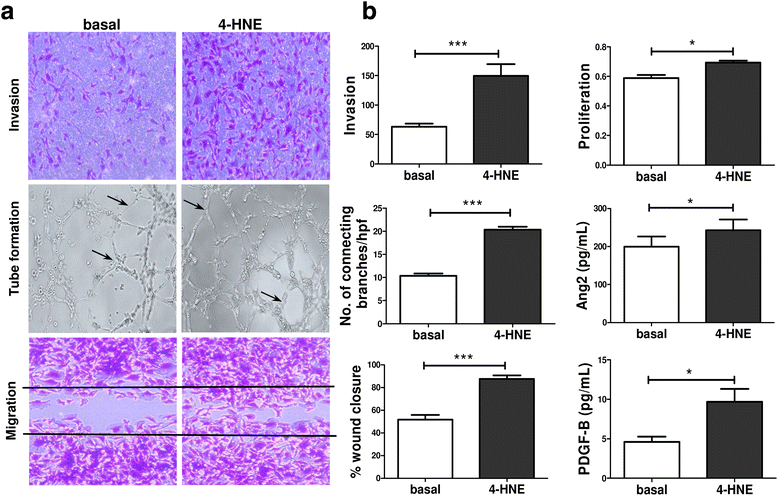
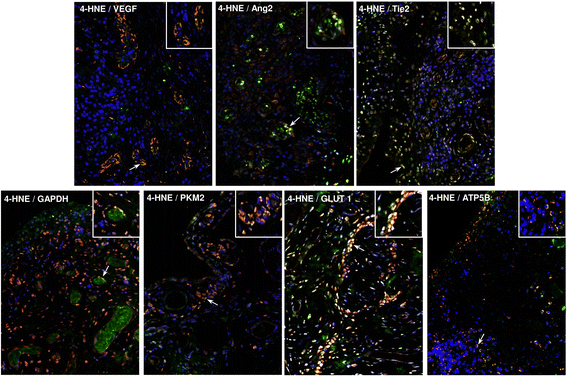
Results: In RASFC and HUVEC, 4-HNE-induced oxidative stress reprogrammed energy metabolism by inhibiting mitochondrial basal, maximal and adenosine triphosphate-linked respiration and reserve capacity, coupled with the reduced enzymatic activity of oxidative phosphorylation complexes III and IV. In contrast, 4-HNE elevated basal glycolysis, glycolytic capacity and glycolytic reserve, paralleled by an increase in mitochondrial DNA mutations and reactive oxygen species. 4-HNE activated pro-angiogenic responses of RASFC, which subsequently altered HUVEC invasion and migration, angiogenic tube formation and the release of pro-angiogenic mediators. In vivo markers of angiogenesis (vascular endothelial growth factor, angiopoietin 2 [Ang2], tyrosine kinase receptor [Tie2]) were significantly associated with oxidative damage and oxygen metabolism in the inflamed synovium. Significant reduction in ST vascularity and Ang2/Tie2 expression was demonstrated in patients with RA before and after administration of TNFi.
Conclusions: Oxidative stress promotes metabolism in favour of glycolysis, an effect that may contribute to acceleration of inflammatory mechanisms and subsequent dysfunctional angiogenesis in RA.
Keywords: Angiogenesis; Bioenergetic metabolism; Oxidative stress; Rheumatoid arthritis.
Conflict of interest statement
Ethics approval and consent to participate
All of this research was carried out in accordance with the Declaration of Helsinki, and approval for this study was granted by the St. Vincent’s University Hospital Medical Research and Ethics Committee. All patients gave fully informed written consent approved by the institutional ethics committee.
Consent for publication
Written consent was obtained from all the participants in and authors of this study.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures



References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

