Dynamic action potential clamp predicts functional separation in mild familial and severe de novo forms of SCN2A epilepsy
- PMID: 29844171
- PMCID: PMC6004444
- DOI: 10.1073/pnas.1800077115
Dynamic action potential clamp predicts functional separation in mild familial and severe de novo forms of SCN2A epilepsy
Abstract
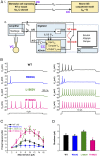
De novo variants in SCN2A developmental and epileptic encephalopathy (DEE) show distinctive genotype-phenotype correlations. The two most recurrent SCN2A variants in DEE, R1882Q and R853Q, are associated with different ages and seizure types at onset. R1882Q presents on day 1 of life with focal seizures, while infantile spasms is the dominant seizure type seen in R853Q cases, presenting at a median age of 8 months. Voltage clamp, which characterizes the functional properties of ion channels, predicted gain-of-function for R1882Q and loss-of-function for R853Q. Dynamic action potential clamp, that we implement here as a method for modeling neurophysiological consequences of a given epilepsy variant, predicted that the R1882Q variant would cause a dramatic increase in firing, whereas the R853Q variant would cause a marked reduction in action potential firing. Dynamic clamp was also able to functionally separate the L1563V variant, seen in benign familial neonatal-infantile seizures from R1882Q, seen in DEE, suggesting a diagnostic potential for this type of analysis. Overall, the study shows a strong correlation between clinical phenotype, SCN2A genotype, and functional modeling. Dynamic clamp is well positioned to impact our understanding of pathomechanisms and for development of disease mechanism-targeted therapies in genetic epilepsy.
Keywords: de novo SCN2A mutation; dynamic action potential clamp; epilepsy; modeling; voltage clamp.
Conflict of interest statement
Conflict of interest statement: G.B. is funded by RogCon, Inc., Miami, Florida, a biotechnology company focused on drug research, discovery, and development for select ion channelopathies, including SCN2A. I.E.S. has served on scientific advisory boards for UCB, Eisai, GlaxoSmithKline, Biomarin, and Nutricia; editorial boards of the Annals of Neurology, Neurology and Epileptic Disorders; may accrue future revenue on pending patent WO61/010176 (filed: 2008): Therapeutic Compound; has received speaker honoraria from GlaxoSmithKline, Athena Diagnostics, UCB, Eisai, and Transgenomics; has received funding for travel from Athena Diagnostics, UCB, Biocodex, GlaxoSmithKline, Biomarin, and Eisai; and receives/has received research support from the National Health and Medical Research Council of Australia, National Institutes of Health, Australian Research Council, Health Research Council of New Zealand, CURE, The American Epilepsy Society, the US Department of Defense Autism Spectrum Disorder Research Program, March of Dimes, and Perpetual Charitable Trustees. S.P. is cofounder, Chief Scientific Officer, and equity holder of RogCon, Inc., Miami, Florida, a biotech company focused on the delivery of novel therapeutics for SCN2A disorders. RogCon, Inc. provided funding for this project. S.P. is also cofounder and equity holder in Praxis Precision Medicines, Inc., Cambridge, Massachusetts, which develops precision medicines for neurogenetic disorders, including those caused by SCN2A mutations. S.P. is a Scientific Advisor and equity holder in Pairnomix, Inc., Minneaopolis, Minnisota, which is undertaking precision medicine development in epilepsy and related disorders.
Figures




References
-
- Heron SE, et al. Sodium-channel defects in benign familial neonatal-infantile seizures. Lancet. 2002;360:851–852. - PubMed
-
- Mantegazza M, Curia G, Biagini G, Ragsdale DS, Avoli M. Voltage-gated sodium channels as therapeutic targets in epilepsy and other neurological disorders. Lancet Neurol. 2010;9:413–424. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

