MINFLUX monitors rapid molecular jumps with superior spatiotemporal resolution
- PMID: 29844182
- PMCID: PMC6004438
- DOI: 10.1073/pnas.1801672115
MINFLUX monitors rapid molecular jumps with superior spatiotemporal resolution
Abstract
Compared with localization schemes solely based on evaluating patterns of molecular emission, the recently introduced single-molecule localization concept called MINFLUX and the fluorescence nanoscopies derived from it require up to orders of magnitude fewer emissions to attain single-digit nanometer resolution. Here, we demonstrate that the lower number of required fluorescence photons enables MINFLUX to detect molecular movements of a few nanometers at a temporal sampling of well below 1 millisecond. Using fluorophores attached to thermally fluctuating DNA strands as model systems, we demonstrate that measurement times as short as 400 microseconds suffice to localize fluorescent molecules with ∼2-nm precision. Such performance is out of reach for popular camera-based localization by centroid calculation of emission diffraction patterns. Since theoretical limits have not been reached, our results show that emerging MINFLUX nanoscopy bears great potential for dissecting the motions of individual (macro)molecules at hitherto-unattained combinations of spatial and temporal resolution.
Keywords: MINFLUX; localization; single molecule; tracking.
Copyright © 2018 the Author(s). Published by PNAS.
Conflict of interest statement
Conflict of interest statement: The Max Planck Society holds patents on selected embodiments and procedures of MINFLUX, benefitting S.W.H., F.B., K.C.G., and Y.E.
Figures
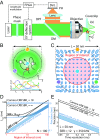

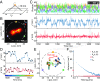
Comment in
-
Monitoring molecular jumps.Nat Methods. 2018 Aug;15(8):572. doi: 10.1038/s41592-018-0091-2. Nat Methods. 2018. PMID: 30065382 No abstract available.
References
-
- Hell SW, Wichmann J. Breaking the diffraction resolution limit by stimulated emission: Stimulated-emission-depletion fluorescence microscopy. Opt Lett. 1994;19:780–782. - PubMed
-
- Betzig E, et al. Imaging intracellular fluorescent proteins at nanometer resolution. Science. 2006;313:1642–1645. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

