Comparison of pediatric allogeneic transplant outcomes using myeloablative busulfan with cyclophosphamide or fludarabine
- PMID: 29844205
- PMCID: PMC5998928
- DOI: 10.1182/bloodadvances.2018016956
Comparison of pediatric allogeneic transplant outcomes using myeloablative busulfan with cyclophosphamide or fludarabine
Abstract
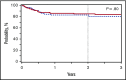
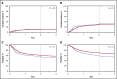
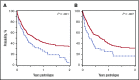
Busulfan combined with cyclophosphamide (BuCy) has long been considered a standard myeloablative conditioning regimen for allogeneic hematopoietic cell transplantation (HCT), including both nonmalignant conditions and myeloid diseases. Substituting fludarabine for cyclophosphamide (BuFlu) to reduce toxicity without an increase in relapse has been increasingly performed in children, but without comparison with BuCy. We retrospectively analyzed 1781 children transplanted from 2008 to 2014 to compare the effectiveness of BuCy with BuFlu. Nonmalignant and malignant disease populations were analyzed separately. Overall mortality was comparable for children with nonmalignant conditions who received BuFlu or BuCy (relative risk [RR], 1.14, P = .52). Lower incidences of sinusoidal obstruction syndrome (P = .04), hemorrhagic cystitis (P = .04), and chronic graft-versus-host disease (P = .02) were observed after BuFlu, but the influence of the conditioning regimen could not be assessed by multivariate analysis because of the low frequency of these complications. Children transplanted for malignancies were more likely to receive BuFlu if they had higher hematopoietic cell transplantation-comorbidity index scores (P < .001) or their donor was unrelated and HLA-mismatched (P = .004). Nevertheless, there were no differences in transplant toxicities and comparable transplant-related mortality (RR, 1.2; P = .46), relapse (RR, 1.2; P = .15), and treatment failure (RR, 1.2; P = .12). BuFlu was associated with higher overall mortality (RR, 1.4; P = .008) related to inferior postrelapse survival (P = .001). Our findings demonstrated that outcomes after BuFlu are similar to those for BuCy for children, but for unclear reasons, those receiving BuFlu for malignancy may be at risk for shorter postrelapse survival.
Conflict of interest statement
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Figures

References
-
- Ozkaynak MF, Weinberg K, Kohn D, Sender L, Parkman R, Lenarsky C. Hepatic veno-occlusive disease post-bone marrow transplantation in children conditioned with busulfan and cyclophosphamide: incidence, risk factors, and clinical outcome. Bone Marrow Transplant. 1991;7(6):467-474. - PubMed
-
- Sencer SF, Haake RJ, Weisdorf DJ. Hemorrhagic cystitis after bone marrow transplantation. Risk factors and complications. Transplantation. 1993;56(4):875-879. - PubMed
-
- Gandhi V, Plunkett W. Cellular and clinical pharmacology of fludarabine. Clin Pharmacokinet. 2002;41(2):93-103. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

